Alkynes – the acidic character of alkynes
Acetylene and other terminal alkynes are weakly acidic in character. They react with strong bases like sodium metal at 475 K or sodamide, to form sodium acetylide derivatives known as acetylides or alkynides with the liberation of hydrogen gas as shown here:
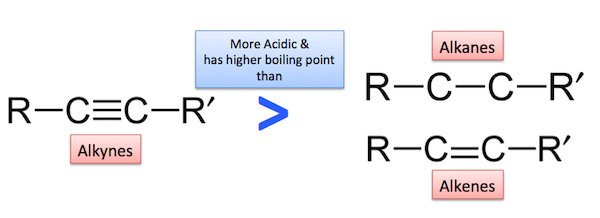
These reactions have not been observed in the case of alkenes and alkanes indicating that alkynes are acidic in nature in comparison to alkenes and alkanes.
Sodium alkynide id decomposed by water regenerating alkyne.
This shows that water is a stronger acid than alkynes and therefore, displaces alkyne from sodium alkynide.
Acetylides react with alkyl halides to give higher alkynes. For example,
The acetylenic hydrogen of alkynes can be replaced by copper or silver ions. They react with ammonical solution of cuprous chloride and ammonical silver nitrate solution to form the corresponding copper and silver alkynides.
Unlike alkali metal acetylides, copper and silver acetylides are not decomposed by water. However, on treatment with dilute mineral acids, they regenerate original alkynes.
It may be noted that heavy metal acetylides are highly explosive when dry. Therefore, these should be destroyed while still wet by warming with nitric acid. It may be noted that only the terminal alkynes react with an ammonical solution of silver nitrate or cuprous chloride. By this, it can be stated that 1-alkynes will give this test while 2-alkynes will not give this test and Therefore, this reaction can be used to distinguish between 2-alkynes and 1-alkynes.
The Explanation for the acidic character: the acidic character of the 1-alkynes can be explained on the basis of the sp hybridization state of carbon atoms in alkynes. As we know that an electron in s-orbital is more tightly held than in a p-orbital because s-electrons are more closer to the nucleus. In sp hybridization, s-character is more as compared to sp2 or sp3 hybrid orbitals. Due to very large s-character, the electrons in sp hybrid orbitals are held more tightly by the nucleus and are quite electronegative. Consequently, the electron pair of the H-C≡ bond gets displaced more towards the carbon atom and helps in the release of a proton. The alkynes with the triple bond in the non-terminal position do not show any acidic character as there is no hydrogen atom attached directly to the triple bonded carbon atom.
Comparison of relative acidic strength of alkanes, alkenes, and alkynes: the alkynes are weakly acidic in nature while alkenes and alkanes do not show any acidic character. This is due to the fact that the carbon atoms of the double bond are sp2 hybridized while those involved in the single bond are sp3 hybridized. Because of lesser s-character, these carbon atoms are much less electronegative than the carbon atoms taking part in a triple bond. Therefore, the release of ion from an alkene and alkane molecule is difficult and they do not show acidic character.