A brief history of the development of the periodic table
The development of periodic table dates back to over 200 century of growth in the understanding of atoms, atomic theory and the discovery of atomic masses of the elements. It was in the year 1869 that Dmitri Mendeleev published the periodic table however his work was based on early discoveries by several scientists such as Antoine Lavoisier and John Newlands. Antoine Lavoisier compiled a list of several basic elements such as oxygen, hydrogen, mercury, nitrogen, phosphorus, sulfur and zinc (Singh, 2011).
Other earlier attempts of classifying elements in groups were as follows:
Prout’s hypothesis, 1815: This hypothesis dictates that the hydrogen atom is considered as the fundamental unit from which all other atoms are made from. This is called the Unitary Theory.
Dobereiner’s Triads, 1829: Dobereiner classified the elements into groups of three with similar properties in a way that the atomic weight of the middle element was the arithmetic mean of the other two. However, he could not arrange all the elements known at that time into his triads of 3 elements.
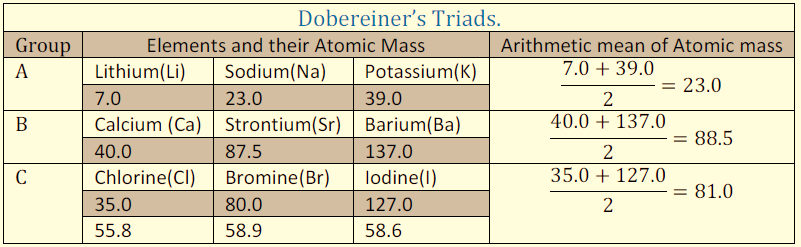
Figure 1: Dobereiner’s Triads
Newland’s Octave, 1864: Newland stated that when elements are arranged in order of increasing atomic mass, every 8th element has properties like the first. This phenomenon is like musical notes having similar arrangements. Newland’s law of octaves contained elements from hydrogen to thorium. Newland assumed that only 56 elements existed in nature and no more elements would be discovered in the future. To fit elements into his table, Newland adjusted two elements in the same slot and put unlike elements under the same note. For instance, Nickel and Cobalt were placed in the same slot and same column as Fluorine, Chlorine and Bromine even though these elements have different properties. However, his arrangement was not valid for elements above calcium (Huheey, Keiter, Keiter, & Medhi, 2006).
Finally, it was Mendeleev’s periodic law that stated that the properties of elements are a periodic function of their atomic masses. His table defined 8 vertical columns called ‘groups’ and 12 horizontal rows called ‘periods’ to accommodate 63 elements. He also left vacant spaces to accommodate the elements to be discovered in future. Likewise, the inert gas elements that were discovered later were placed in a separate group without disturbing the table. However, some elements in Mendeleev’s periodic table were not arranged in increasing order of their atomic masses. The position of hydrogen was uncertain as it portrayed both metallic and non-metallic properties and position of isotopes was also not made clear. In some cases, the wrong order of elements was not justified. For example, Argon (39.9) was placed before Potassium (39). Moreover, since isotopes were discovered long after Mendeleev proposed his classification, they posed quite a challenge to Mendeleev’s Periodic Law. Incidentally, this table helped in predicted three other elements during its time. These elements were named scandium, gallium and germanium. The properties of these elements closely corresponded with Mendeleev’s prediction.
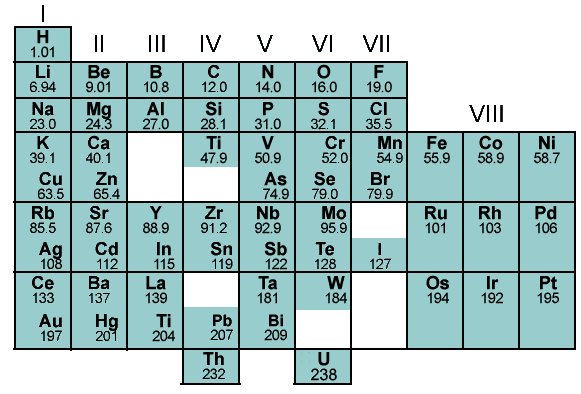
Figure 2: Mendeleev’s periodic table