Chemical Thermodynamics: Heat
Heat and the thermodynamics form the basics which helped process designers and engineers to optimize their processes and harness the energy associated with chemical reactions economically. When researchers especially researched on thermodynamics, they were only researching heat and thermal energy. Heat is able to do anything: I can flow from one area to another, get atoms empowered, and even maximize energy.
Heat in thermodynamics is defined as the kinetic energy of molecules in a substance that in turn increases its internal energy. Heat energy goes from maximum temperature to minimum temperature. Heat added to a system is given by a positive sign, whereas heat extracted from a system is given a negative sign. It is an extensive property.
In fluids, heat is transferred by convection, in which the motion of the fluid itself carries heat from one place to another. An extra way to move heat is by doing conduction, where no motion of a substance is involved, but moving energy undergoing a substance (or besides substances in contact) takes place. The third option to move energy is by radiation, which inculpate absorbing or spreading off electromagnetic waves.
Heat moves from one system to another due to the differences in the temperatures of the systems. If two identical systems with equal temperatures are present, no flow of energy occurs. When two systems with different temperatures are present, the energy will start to flow. Air mass of high pressure enable the force molecules in large numbers into areas of minimum pressure. Places of maximum temperature spread off energy to places with minimum temperature
When there are two systems and both are in thermal equilibrium, there is no basic heat moves between them. This occurs when the systems are at the same temperature which means that the systems at the same temperature will be in thermal equilibrium with each other.
Temperature is a calculation of the basic or average kinetic energy. This is especially important for liquids and solids because the kinetic energy is almost entirely vibrational and rotational. All molecules above a temperature of absolute zero are in a continual state of motion and possess kinetic energy. The Kelvin is the high-end international unit for temperature.
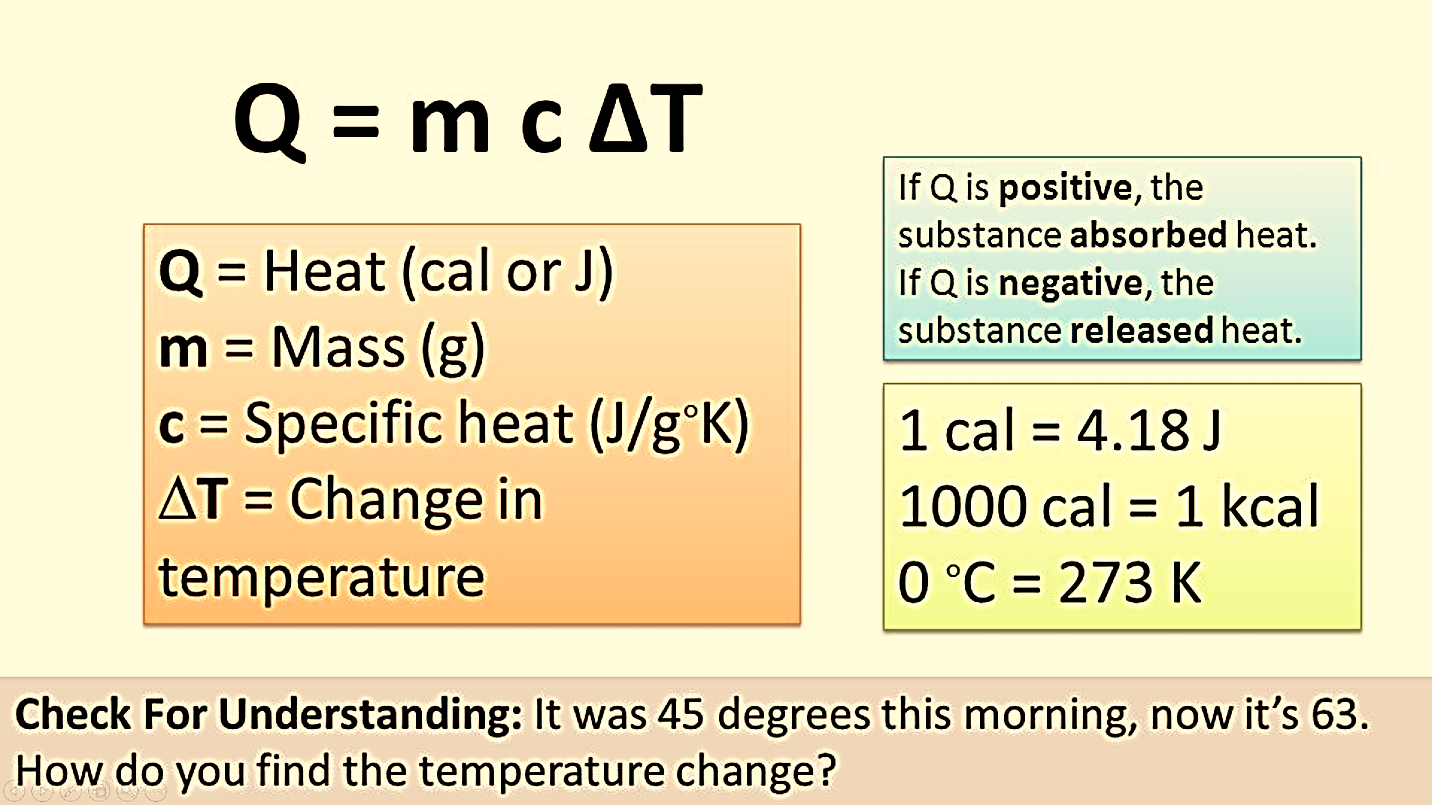
Figure 1: Relationship between temperature and heat.
From the table below, the isobaric process is one where the pressure of the system remains constant. Both the volume and temperature change. The isothermal process is one where the temperature of the system remains constant; therefore, by the ideal gas laws, the product of the volume and the pressure remains constant. An adiabatic process is one where there is no heat exchange with the outside world. An isochoric process is one where the volume of the system remains constant as the pressure and temperature change. In each case, the work done is the area under the curve. Note that no work is done in the isochoric process.
Table 1: Important thermodynamic processes for an ideal gas
| Process | Characteristic | Result | Equation | PV graph |
| Isothermal | T= constant | ∆U = 0 | Q = W | 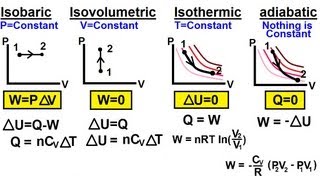 |
| Isobaric | p= constant | W=p∆V | Q = ∆U =p∆V | 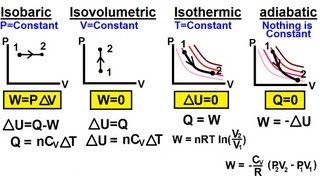 |
| Isochoric/
Isovolumetric |
V= constant | W = 0 | ∆U = Q | 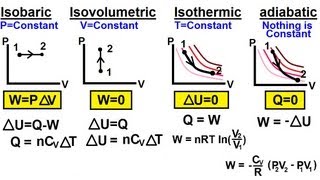 |
| Adiabatic | Q=0 | ∆U = -W | 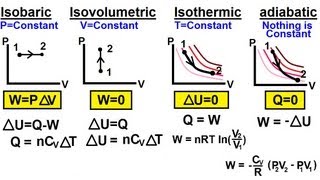 |