Generally, alkenes are easily oxidized with a large number of oxidizing agents. In the presence of oxygen, alkenes are burnt with a bright flame and produce water and carbon dioxide. Epoxides are produced by the catalytic oxidation with the per-carboxylic acids. In the process of ozonolysis, the reaction of alkenes with the ozone causes the breaking of the double bonds and yields the two ketones or aldehydes. The reaction of alkenes with the hot and concentrated potassium permanganate in the acidic solution yields the carboxylic acids or ketones. This reaction is also useful for the determination of the position of the double bond in an unknown alkene. At the vicinal diol, the oxidation can be stopped, rather than the full cleavage of the alkenes by using the diluted potassium permanganate of low temperature or by using osmium tetroxide or some other oxidants.
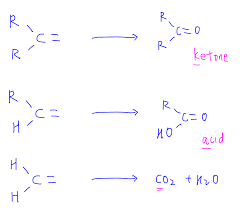
Alkenes are the unsaturated hydrocarbons and their reactive site is the double bond. Under the mild oxidizing conditions, the double bond of alkenes cannot be easily broken. When oxidation occurs under the mild oxidizing conditions, then it produces the diols and these reactions are referred to as hydroxylation reactions. When the oxidizing conditions are strong then they easily break the double bond and these strong oxidizing conditions are ensured by the presence of concentrated, hot potassium permanganate. Under these conditions, ketones and carboxylic acids are produced. If a methylene compound is to be oxidized then the products will include the water and carbon dioxide gas. Some examples of strong oxidation are as follows.
- The conversion of ethene or ethylene to the water and carbon dioxide.
- The oxidation of propene and propylene to the acetic acid also called ethanoic acid by yielding water and carbon dioxide.
- The oxidation of 2 methyl-propene or 2 methyl-propylene to the acetone by producing carbon dioxide and water.
At cold temperatures and with the low concentrations of the oxidizing agents’ alkenes makes the glycols. Alkenes also react with the cold diluted solution of potassium manganate. Sometimes, this reaction is known as the Baeyer test as potassium permanganate which is purple is reduced to produce the manganese dioxide that is brown precipitate. Any compound that is soluble in water should produce this change of the color when it is added to the cold potassium permanganate and should possess the double or triple bonds. In this reaction syn addition is involved which leads to the formation of the cis-glycol. The cis-glycol can also be produced by the reaction of an alkene with the osmium tetroxide.
By using the higher temperatures and the concentrated solution of the potassium permanganate, the glycol is further oxidized and it causes the formation of the mixture of carboxylic acids and ketones.
Alkenes are also oxidized with the ozone and it destroys both pi and sigma bonds of the system. Generally, this cleavage is accomplished in the good yield and is known as ozonolysis and its resulting products are ketones and the aldehydes. By the identification of the products obtained from the reaction, the isomers can be distinguished from each other and the position of the bonds in the original compounds can be easily determined.