Group 13 elements – a variation of properties
Let’s have a look at the variation of physical and atomic properties of the group 13 elements along the periods and the group.
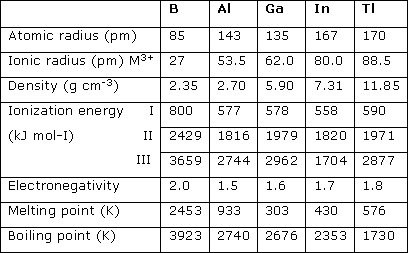
- Atomic and ionic radii: The atomic and ionic radii of group 13 elements are smaller than the group 2 elements. Because as we move from left to right in a periodic table nuclear charge increases, but electron is being added into the same shell. This same shell electron does not provide enough shielding effect and effective nuclear charge increases. As a result, size decreases.
While on moving down the group atomic and ionic radii should increase as expected because electron is now getting added into a new shell. But there is one exception as we move from Al to Ga radius slightly decreases. This is because while we go from Al to Ga there are also ten d-electron. These d-electrons do not screen the nucleus properly because of their poor shape and penetration power and effective nuclear charge in Ga becomes more and size decreases a bit. - Ionization enthalpy: As we go from left to right ionization enthalpy increases. But the first ionization enthalpies of group 13 elements are less than corresponding members of group 2. We know that the first electron in the case of group 13 element is to be removed from p-orbital while in the case of group 2 it has to be removed from s-orbital. Since the p-orbital are at a slightly higher energy level than s-orbital hence they are weakly held by the nucleus and hence the first ionization enthalpy is less. However, second and third ionization enthalpy is high as expected.
On moving down the group ionization enthalpy decreases as a trend. But in group 13 there is a sharp decrease in enthalpy from B to Al but as we move to Ga first ionization enthalpy is slightly higher than of Al. then again it decreases from Ga To In and first ionization enthalpy of Tl is higher than Al, Ga, In. - Electronegativity: As per the trend electronegativity increases as we move from left to right and decreases as we move down along the group. The electronegativity of group 13 elements is higher than group 1 and group 2 elements. In group 13, electronegativity first decreases from B to Al and then increases down the group. Because as we move down the group, B to Al atomic size increases considerably. Therefore, the attraction of the nucleus for electrons decreases and hence electronegativity decreases. On further moving down the group, although the size increases, yet the effective nuclear charge increases because of the poor shielding effect of d- and f- electrons. As a result, the force of attraction of the nucleus for the electrons increases and hence the electronegativity increases from Al to Tl.
- Melting and Boiling point: The melting and boiling point of group 13 elements decreases as moving down the group. However, the decrease in the melting point is not as regular as in boiling point.
- Electropositive or metallic character: Due to the high ionization enthalpy of group 13 elements they are less electropositive as compared to group 2 elements. On moving down the group, the electropositive character increases because ionization energy decreases. For example Boron is a non-metal while other members of the group are typical metal.