Isomerism of Alkanes is the property exhibited by the alkanes. It is depicted when the molecular formula of the compound is the same but there are structural differences. Due to this phenomenonhabited by the compounds the chemical properties if the compound also shown differences.Isomerism is shown by a number of compounds.
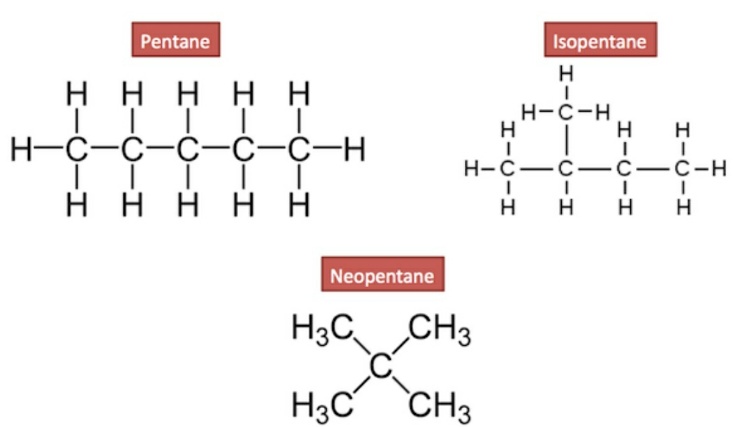
Generally, the isomers coexist together so as to maintain stability in the compound. The first 3 members of alkane are methane, ethane and propane have only one structure. But above the 3rd member of the alkane family,the alkanes show the property of isomerism.
As the number of carbons increases in the structure, the number of isomerism of alkanes exhibited by the compound also increases. Basically, the isomerism of the alkanes is divided into different categories. The isomerism can be divided into Constitutional Isomers and Stereoisomers.
The constitutional isomers are also known as Structural isomers. In this type of isomerism,the molecular formula of the alkanes is same but the atomic organization is different. Also, the bonding patterns of the structureis different. For example, in the above structure of pentane just by differing the type of pattern of binding and organization of atoms we get three different forms of isomers. In pentane structure, the bonding of the carbon is normal in a single chain and the parent member is pentane. If one CH3 carbon is attached on the second carbon of the parent chain them we get a new isomer known as Isopentane. If four CH3 methyl atoms are attached on one atom of carbon then the obtained product of isomer is Neo pentane.
The stereoisomers are also known as spatial isomers. In the world of isomerism of alkanes of Stereochemistry, the stereoisomerism is referred to as the type of isomerism In which the molecular formula of the structure is the same and the bonding pattern of the atoms is also the same. The inky thing differing in the stereo isomers is the 3D orientation of the structures. The orientation of the structure in the threedimensions differs the chemical properties of the structures and thus depicting isomerism.
The stereoisomers are further classified into enantiomers and diastereomers. The diastereomers are further classified into cis and trans isomers and conformers and rotamers.
Enantiomers are types of optical isomers that are the mirror images of each other. These are types of enantiomers that are always existed in pairs. The enantiomers isomers never superimpose on each other. This is because they are mirror images of each other.
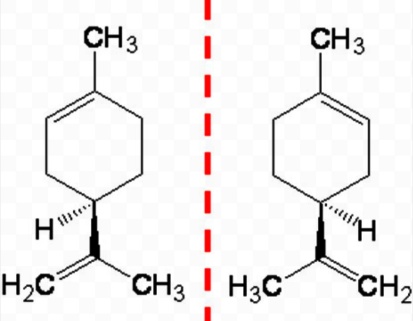
The diastereomers are a type of stereoisomers that are non mirror image isomers. These are also a type of non identical stereoisomers.In the diastereomers, there are two types of isomers. They include cis type diastereomer and trans type diastereomer. In the cis type diastereomer both the identical atom lie on one side of the structure. Whereas in the trans type diastereomer the identical atoms lie on the opposite side of the structure. Different type of diastereomers depicts different types of properties. Generally the trans and cis isomers co-exists but in different proportionate. There are further classifications of isomersofalkanes and different methods are used to determine their proportions and types.