Specific and molar conductivity
Here is the description of molar conductivity and specific conductivity. Material’s conductance is material’s property because of which a material lets the ion’s flow going through itself and therefore, conducts electricity. Generally, it’s described as resistance’s reciprocal of that particular material. S (Siemens) is the SI unit of conductance. Conductivity or well-known as Specific Conductivity is the measurement of material’s ability for conduction of electricity. ‘K’ is the symbolic representation of it. As a result, by definition,
G = 1 / R
R= ρ * 1 / A
К= 1 / ρ
G =К * A / l
Where,
ρ = resistivity of the material
К = conductivity,
G= conductance
A= area of cross section
l = length
R= resistance
Or,
Specific conductance also known as the conductivity.
Moreover,
![]()
![]()
![]()
Or,
Specific conductance/conductivity = cell constant × Conductance
Materials’ conductance is dependent on materials’ nature, temperature, and the number of valence electrons belonging to the material. Metals are known to be good conductors of electricity because of the valence electrons present in them. As per observation, materials’ conductance goes down with the rise in temperature.
Water when in pure state, is known having a very low conductivity because of hydroxyl ions’ presence. Electrolytes’ presence moreover strengthens conductivity as they enhance their ions in a solution.
The electricity’s conductance by the ions are present in the solution which is known as ionic or electrolytic conductance. The conductivity or specific conductivity of the electrolyte solution at any known concentration refers to the conductance of 1 unit volume of solution retained between 2 platinum electrodes accompanying cross-sections’ unit area and at the distance of the unit length. Electrolytic solutions’ specific conductivity is dependent on:
- Temperature.
- The concentration and nature of electrolyte which is added
- Temperature
- Ion’s size produced plus their salvation
- Solvent viscosity and its nature
Because of charge, the size and the concentration of the ions in which the electrolytes eases or dissociates with which there’s movement of ions under conductivity and the potential gradient of solutions of various electrolytes varies with identical solvent and at a defined temperature.
As a result, we describe a much general term called, the molar conductivity for the electrolyte solution.
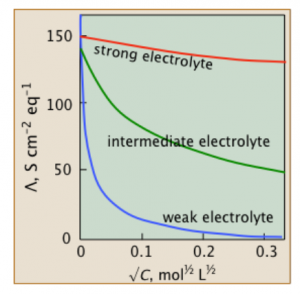
The image shows the molar conductivity for different electrolytes
At a given concentration, solution’s molar conductivity is the conductance of solutions’ volume which contains 1 mole of electrolyte placed between 2 electrodes with cross sections’ unit area and unit length’s distance. Generally, it’s illustrated as the ratio between electrolyte’s concentration and specific conductivity. The symbol Ʌm indicates it.
Ʌm = Kc
Where,
К = Specific conductivity
c= Electrolyte’s concentration
Molar Conductance
The conduction of all ions generated by the ionization process of 1-gram mole of the electrolyte, present in V (mL) of solution, is known as the molar conductance. The denotion of it is given by:
Molar conductance
μ = k ×V
Where,
V = volume in mL which contains electrolyte of 1 gram mole,
Also, if c = solution’s concentration in gram mole/litre, then,
Where,
V = volume of electrolyte (mL) which contains 1 g mole.
And If c = Solution’s concentration in actual g sesame, then
μ = k × 1000/c
Molar conductance’s unit: ![]()
Equivalent conductance = (Molar conductance) / n
where,
n = (Molecular mass) / (Equivalent mass)