Alkenes are the members of the family of the hydrocarbons. In between their carbon atoms, there is a double bond. Akenes can be prepared by the various methods and in this article, some of the methods will be discussed.
Preparation of Alkenes from Alkynes
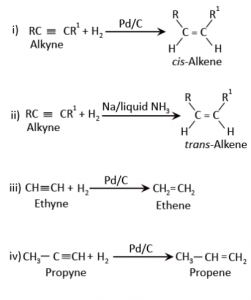
Alkenes can be prepared by using the alkynes. The conversion of the alkynes to the alkenes is carried out by the reduction of the alkynes with the hydrogen in the presence of the palladised charcoal. This charcoal is moderately deactivated with the help of sulfur compounds or the quinoline. Resultantly, this reaction causes the formation of the alkenes. The deactivated charcoal that is used in this process is also called as the Lindlar’s catalyst. The alkenes obtained by this process have the cis-geometry. For making the trans-alkenes, alkynes undergo a reduction in the sodium in the presence of the liquid ammonia.
Preparation of Alkenes from the Alkyl Halides
Alkenes can also be produced by heating the alkyl halides with the alcoholic potash. Dehydrohalogenation takes place in this reaction as one molecule of the halogen acid is removed. The rate of this reaction is dependent on the nature of the halogen group attached and the alkyl group. The process of dehydrohalogenation is proceeding by the following mechanism.
• A slightly acidic proton of the hydrogen is removed by the strong base from the alkyl halide by using an acid-base reaction.
• The electrons which are released due to the breaking of the hydrogen-carbon bond are attracted to the carbon that is slightly positive and is attached to the chlorine atom. The approach of these electrons to another carbon causes the hydrogen atom to break free and it leads to the formation of the double bonds.
Preparation of Alkenes from the Vicinal Halides
In the vicinal dihalides, the two halogens are attached to the two adjacent carbon atoms. The reaction of these dihalides with the zinc metal causes the loss of halogen molecule and alkenes are formed. This reaction to prepare the alkenes from the vicinal dihalides is called the dehalogenation.
Preparation of Alkene from Alcohols
The reaction of alcohols with the concentrated sulfuric acid causes the formation of the alkenes by the elimination of the water molecule. Due to the removal of the water molecule, this reaction is called acidic dehydration of alcohol and here concentrated sulphuric acid is used as a dehydrating agent. Generally, the heating of most of the alcohols is the common method for the preparation of the alkenes.
The mechanism for this reaction includes few steps such as protonation of alcohol, dissociation of oxonium ion, and deprotonation of carbonation. At the high temperatures, this reaction is more favored and the most important acids in the laboratory used for this purpose are phosphoric acid and sulphuric acid which are termed as the Bronsted acids as they are donors of the protons.
Kolbe’s Electrolysis
Alkenes can also be produced by the electrolysis of the aqueous solution of potassium or the sodium salts of the saturated dicarboxylic acids.