The electronic configuration of an atom is the represents the arrangement of the electrons distributed among the shells and subshells. In other words, electronic configuration describes how the electrons are assembled in the shells and subshells of the atoms. Commonly it is used for describing the orbits of an atom in the ground state but it can also be used for representing any atom that has been ionized into the anion or cation by compensation of gain or loss of the electrons in the subsequent orbits of the atom. Due to the unique electronic configuration, many of the physical and chemical properties of the elements can be correlated. Valance electrons which are in the outermost shells, mainly determine the unique chemistry of the elements.
S Block Elements Group I and Group II
Those elements in which the inner block shells are completely filled and the last electron enters the s orbit of the outer most shell are called s block elements. So, for these elements, the differential electron enters to the ns orbital. Hence, the outermost electronic configuration of the s block elements is ns1 or ns2 as all the inner orbitals are completely filled. In the modern periodic the s block elements have been placed in the first two groups that are the group I (alkali metals) and group II (alkaline earth metals). S block is filled by the principal quantum number “n”. Maximum there are two electrons that can occupy the s block.
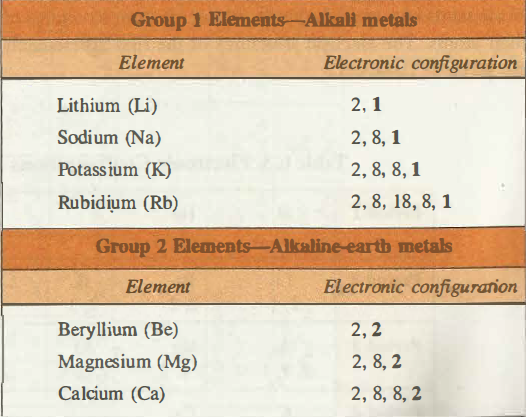
Electronic Configuration of Group I Elements
Electronic configuration represents that the ultimate shells of the alkali metals do have one s electron (S1 electron) while in the penultimate shells there are eight electrons S2P6 except for the Lithium. When the electron in the valance shell is lost then these alkali metals are converted into the M+ ions whose configuration is that of inert gas. As the additional electron enters the ns-orbital so these elements are the s block elements.
Electronic Configuration of Group II Elements
The general electronic configuration for the group II elements is ns2. The electronic configuration for Beryllium is 1s2 2s2 for Magnesium it is [Ne]3s2, for Calcium [Ar]4s2, for Strontium [Kr]5s2, and for Radium it is [Rn]7s2. In this group, the outer electrons feel the pull of +2 charge from the nucleus, i.e. the number of protons offset by the number of the inner electrons.
The outer electrons of all of the elements in this group experience a force of net charge of +2 from the center of the nucleus. In the nucleus, this positive charge is balanced by the negativity of the inner electrons. The same happens to all the atoms in this group. The number of layers of the inner electrons is the only factor that influences the size of the atoms. When electron layers are more, more space is taken by them as repulsion occurs due to the nearest electrons so the size of the atoms grows as we move down to the group.