What is the Van’t Hoff Factor?
The Van’t Hoff factor ‘i’ gives you the introduction on effects of the solutes on solutions’ colligative properties. It is represented by the symbol ‘i’. The Van’t Hoff factor can be outlined as ratio of formation of particles’ concentration when the substances dissolves to substance’s concentration by mass/weight.
The magnitude up to which the substance dissociates or associates in the solution, it’s described by Van’t Hoff factor. For instance, When the substance non-electrolytic in nature in water is dissolved, the ‘i’ value is usually one. Nevertheless, when the ionic compound in water forms a solution, then the ‘i’ value is equal to number of ions present in total amount in a single formula unit of substance.
Let’s say, CaCl2’s Van’t Hoff Factor is theoretically 3, since the compound of CaCl2 dissociates into two Cl– ions and one Ca2+ion. Nonetheless, a few of the ions associate with one another in a solution, which leads to the decrement in total particles’ number in a solution.
Association or dissociation effect of the solute on a solution and the Van’t Hoff factor is given in tabulated form:
Association Dissociation
‘i’ is less than one ‘i’ is greater than 1
Van’t Hoff in 1886 brought the factor ‘i’ which is well-known as Van’t Hoff’s factor. To express the range of dissociation or association of the solutes in a solution. It is expressed as the ratio of observed and normal molecular masses of the solute, which is:
![]()
In association case, the observed molecular mass is more compared to the normal one and the value of Van’t Hoff factor, ‘i’ is lesser than 1. But when we take dissociation into consideration, then ‘i’ is more than 1 as the normal molecular mass has a greater value than observed molecular mass. Just in case, there’s no dissociation, the value of Van’t Hoff ‘i’ is equal to 1.
As known, molecular masses and colligative properties are inversely propotional to each other, the ‘i’ factor can also be expressed as:
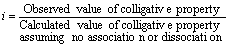
or,
![]()
Van’t Hoff factor’s introduction changes colligative properties’ equations as followed:
The Relative lowering of vapour pressure 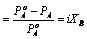
Boiling point Elevation, ∆Tb = i * K.bm
Freezing point Depression, ∆Tf = i * Kf * m
Osmotic pressure, π = inRT / V ; π = i * CRT
From the ‘i’ value, it’s possible to calculate dissociation degree or the association degree of a substance.
Dissociation Degree (a): Dissociation Degree is known as the total molecules’ fraction that dissociate themselves into simpler ions or molecules.
Where, m= number of particles present in solution
Association Degree (a): Association Degree is known as fraction of molecules’ total number which combines together or associate and results to form bigger molecules.
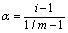
Where, m = number of particles present in solution.