Qualitative Analysis
Qualitative method is an analytical method which is used to determine the number of elements or molecules produced during a chemical reaction. Organic compound comprises of Carbon, Hydrogen, Oxygen, Nitrogen, phosphorus, sulphur and halogens. Various methods to determine the per cent composition of element in the organic compound are explained below.
Detection of Carbon and Hydrogen
Carbon and Hydrogen are detected by heating the compound with Copper II oxide in a hard glass tube. Carbon is oxidised to CO2 and Hydrogen is oxidised to H2O. If the lime water turns milky due to CO2, and the anhydrous CuSO4 turns blue due to H2O turns blue, then the presence of Carbon and Hydrogen is confirmed.
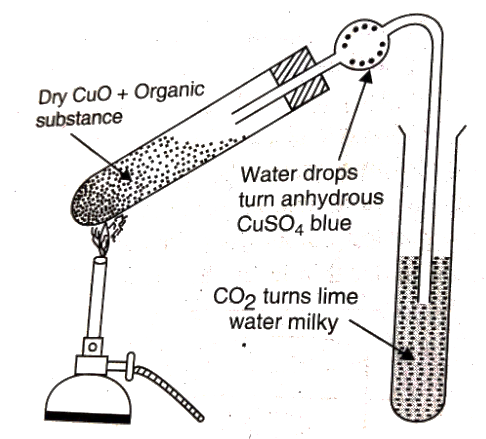
Fig: Detection of Carbon and Hydrogen
Detection of other elements
Sodium Fusion extract: A small piece of sodium metal is heated along with an organic compound in fusion tube for 2-3 minutes. The red hot tube is plunged in to distilled water contained in china dish. The filtrate is called as sodium fusion extract.
Test for Nitrogen: The sodium fusion extract is boiled along with iron II sulphate. It is then acidified with concentrated sulphuric acid. The formation of Prussian blue colour shows the presence of Nitrogen
6CN– + Fe2+ → [Fe (CN) 6] 4-
3[Fe (CN) 6]4- + 4Fe3+ ———– Fe4 [Fe (CN) 6] 3.xH2O
Test of Sulphur: The sodium fusion extract is acidified with lead acetate and acetic acid is added to it. The presence of sulphur is confirmed by the black precipitate of lead sulphide.
S2- + Pb2+⎯→PbS
Test of halogens: The sodium fusion extract is acidified with nitric acid. After this it is treated with silver nitrate.
White precipitate soluble in ammonium hydroxide ———- Presence of chlorine
Yellowish precipitate sparingly soluble in ammonium hydroxide——— Bromine
Yellowish precipitate sparingly insoluble in ammonium hydroxide——— Presence of iodine
X–+ Ag+⎯→ AgX (Here X represents a halogen)
Quantitative Analysis
Carbon and Hydrogen
Percentage of carbon= 12 x100xm2/ 44 m
Percentage of hydrogen = 2 x m1 x100/ 18 m
Where m, g= mass of organic compound.
m1 and m2 g respectively= Mass of water and carbon dioxide produced be;
Nitrogen (Dumas Method): A known mass of organic compound, when heated with copper oxide (CuO) in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water.
Volume of nitrogen = p1 V1 x 273/760 x T1
Kjeldahl’s method: A known mass of organic compound is heated with concentrated H2SO4 in presence of K2SO4 and little CuSO4 or mercury in long necked flask called kjeldahl’s flask. Nitrogen present in the compound gets converted into ammonium sulphate. The resulting acid mixture is then heated with excess of sodium hydroxide solution to liberate ammonia.
The volume of acid unused is found by titration against a standard alkali solution. The per cent of nitrogen is determined by applying the following equation:
Halogen (Carius Method) : Halogen containing organic compound is heated with fuming nitric acid in the presence of silver nitrate. Silver halide (AgX) is formed by the halogen present. It is filtered, washed, dried and weighed.
Mass of halogen in m1g of AgX = Atomic mass of X * m1g / molecular mass of AgX *m
Here, m g = mass of organic compound taken
Mass of AgX formed = m1 g