The definition of Raoult’s law says that the solvent’s vapor pressure above the solution is equivalent to pure solvent’s vapor pressure at the same temperature which can be measured by the solvent’s mole fraction, it’s represented as:
Psolution = χ solvent * Po solvent…….. (1)
Raoult’s Law Regarding Volatile Solutes
Raoult’s law states that in a solution, the vapor pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the vapor pressure of that component in the pure state.
Raoult’s rule states that in one solution, the vapor pressure of one component at the given temperature is equal to the fraction of the component which is multiplied by the vapor pressure of that component in pure condition.
Raoult’s Law for Non-Volatile Solutes
The statement of Raoult’s law says that the relative vapor pressure’s lowering of the solution which has a non-volatile solute is the same as the solute’s mole fraction in a solution.
Raoult’s Law is expressed by the formula:
Psolution = ΧsolventP0solvent
where,
Psolution = vapor pressure of a solution
Χsolvent = mole fraction of a solvent
P0solvent = vapor pressure of a pure solvent
If solute, more than one is added to a solution, then the individual component of each solvent get an add-on to the net pressure.
Raoult’s law is similar or connected to an ideal gas, other than the solution. The ideal gas law presumes the ideal behavior in which the intermolecular forces between not alike molecules is equal to the forces between the identical molecules. Also, Raoult’s law presumes that the component’s physical properties of the chemical solution are similar.
Deviations From Raoult’s Law
If there are cohesive and adhesive forces between the two liquids, deviations are observed from Raoult’s law.
This behavior is observed in the mixture of chloroform and acetone. Here, hydrogen bonds cause deviation. Another example of negative deviation is in hydrochloric acid and water solution. Here, the hydrogen bond is the reason for the deviation. One more example of negative deviation is solution of water and hydrochloric acid
The Positive deviation takes place when cohesive force between molecules identical molecules are more than the adhesive force, contrary to molecules. The outcome is high than the vapor pressure which is expected.
Both mixture’s components escaped the solution more quickly comparative to the components that were pure. This kind of behavior is seen in the mixtures of methanol and benzene and the mixtures of ethanol and chloroform.
This behavior is observed in the mixture of benzene and methanol and in the mixture of chloroform and ethanol.
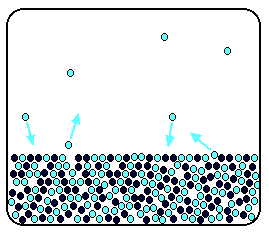
Fig: Dynamic equilibrium of the solution having non-volatile solute molecules in black circles and volatile solvent molecules in teal circles. Observe that a few of the molecules are present in the gaseous phase in comparison to the pure solvent in Fig
Limitations on Raoult’s Law
In reality, there’s not at all such type of thing as the ideal solution! Nevertheless, the characteristics of one include:
- The ideal solutions meet the needs of Raoult’s law. The solution in Figure 1 mentioned above will not really follow Raoult’s law – it is very much concentrated, but was made so concentrated to focus the point.
- In the ideal solution, it exactly takes the same energy amount for the molecule of the solvent to break out from the solution’s surface as it occurred in pure solvent. The attraction forces present between solute and solvent are accurately the same as between the original molecules of the solvent.
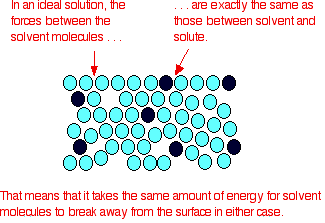
Fig