Chemical Thermodynamics: Enthalpy of bond dissociation
Bond enthalpy can be used to calculate unknown enthalpy changes for a reaction. However, there are limitations to the results of these calculations as they are based on average bond enthalpies (i.e. typical values for a bond) and only valid gaseous reactants AND products
The bond dissociation enthalpy/energy (BDE) is the energy required to break 1 mole of a specified bond for a gaseous species at 298K/25°C.
For example, the bond energy of an O-H single bond is 463 kJ/mol. O-H bond requires 463 kJ of energy to break one mole of O-H bonds. To break any bond, energy must be absorbed. Hence, bond breaking is always an endothermic change (ΔH > 0) but energy is always released upon the formation of a bond. Therefore, bond making is an exothermic change (ΔH < 0). Change in reaction enthalpy (ΔHrxn) can be approximated using the bond energy data. We may assume that during a chemical change, all bonds in the reactants are broken yielding free atoms or radicals. These atoms recombine to form the new bonds present in the products.
The change in enthalpy for a given reaction is equal to the combined total of the energy required to break any bonds that are broken and the energy released from any bonds that are formed.
ΔHrxn= ΣΔHbreaking + ΣΔHmaking
The equation above can be defined as the sum of the bond enthalpies of the broken bonds minus the sum of the energies of the bonds that are formed (forming bonds releases energy). An advantage of this method is that the bonds that are changing in a given reaction are taken under consideration.
It is important to understand that bond dissociation energy has a positive sign because the energyis required and not released. Also, it is the energy for one mole of a bond and not for one mole of a substance.
Likewise, bond dissociation energy is obtained a zero Kelvin by spectroscopic methods. In diatomic molecules, the bond dissociation energy is equal to the value of bond energy. The bond between fluorine and silicon is said to have the strongest bond dissociation enthalpy due to the valency of silicon. One point to note is that covalent bonds between atoms or molecules have weak bond dissociation energies than metallic bonds.
For example, hydrogen, H2 (H-H),
H2(g) 2H(g) Bond energy = +436 kJ
Consider a polyatomic molecule, for example, methane, CH4.
Methane has four identical C-H bonds, but each of these bonds has a different BDE (only approx. values are shown below, but they are different).
CH4(g) CH3(g) + H(g) BDE= +440 kJ
CH3(g) CH2(g) + H(g) BDE= +460 kJ
CH2(g) CH(g) + H(g) BDE= +420 kJ
CH(g) C(g) + H(g) BDE= +340 kJ
The strength of each individual C-H bond is somewhat different. In order to simplify this, we define the bond energy as the average of the four C-H bonds.The mean bond enthalpy is the average bond dissociation enthalpy for a bond in a range of different compounds.
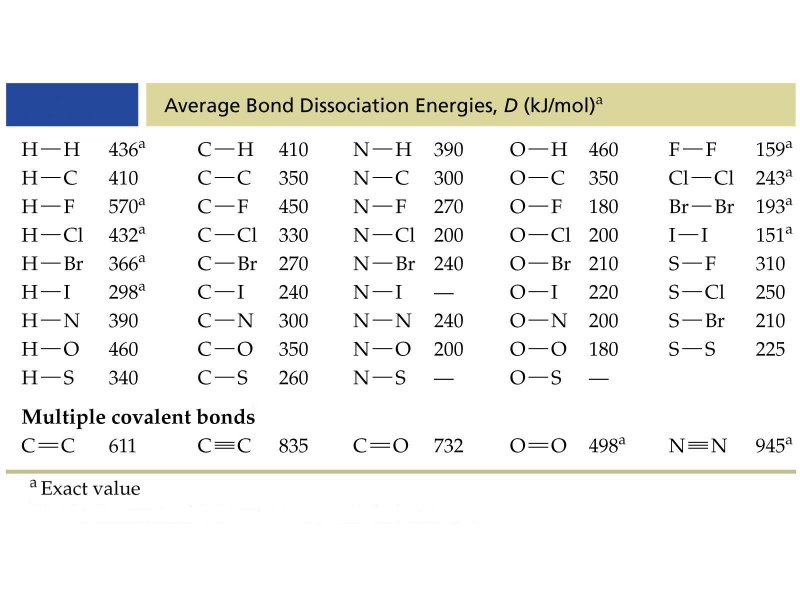
Figure 1: Bond dissociation energy values for some molecules