Surface Chemistry: Electrophoresis
Electrophoresis means “to carry with electricity”. It is a method where charged molecules in solution, migrate in response to an electrical field. Their rate of migration through the electrical field, depends on the strength, net charge, size, and shape of the molecules, along with the ionic strength, viscosity, and temperature of the medium through which the molecules are passing. As an analytical tool, this process is simple, robust and highly sensitive. It can be used analytically to study the properties of a single charged species and separate mixtures of molecules.
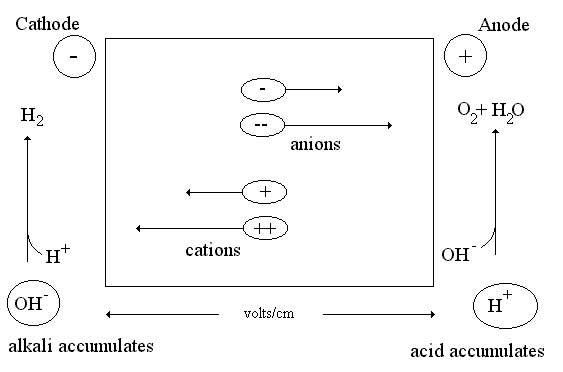
Fig1: Schematic diagram of the electrophoretic cell.
The movement of dispersed phase particles in the electric field is called electrophoresis. The rate of movement depends on the ζ-potential value. Electrophoresis is widely used for aminoacids, antibiotics, enzymes, antibodies and other objects separation. It is also used for plasma proteins investigations with diagnostic purposes. Proteins are amphoteric compounds containing both acidic and basic residues. Each protein has its own characteristic charge properties depending on the number and kinds of amino acids carrying amino or carboxyl groups. Nucleic acids, unlike proteins, are not amphoteric.
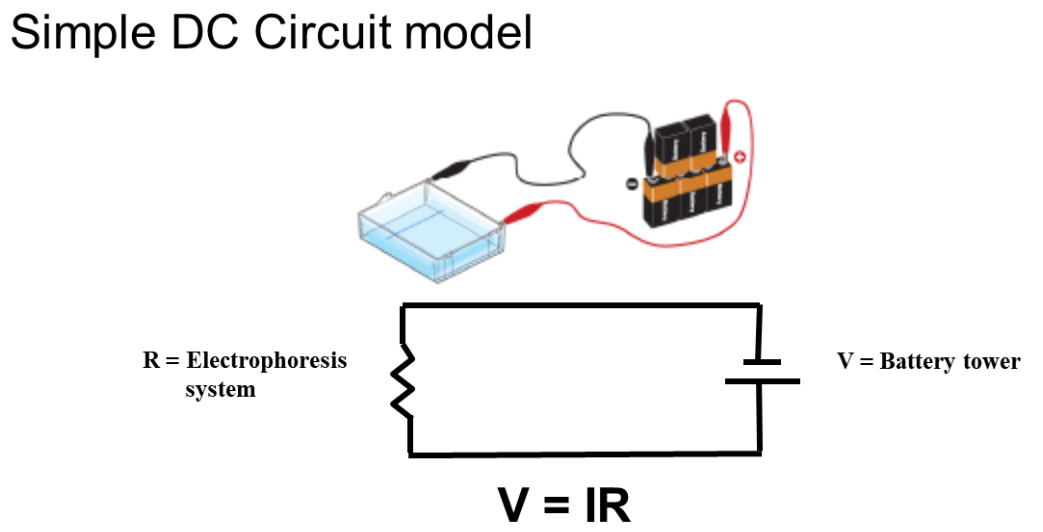
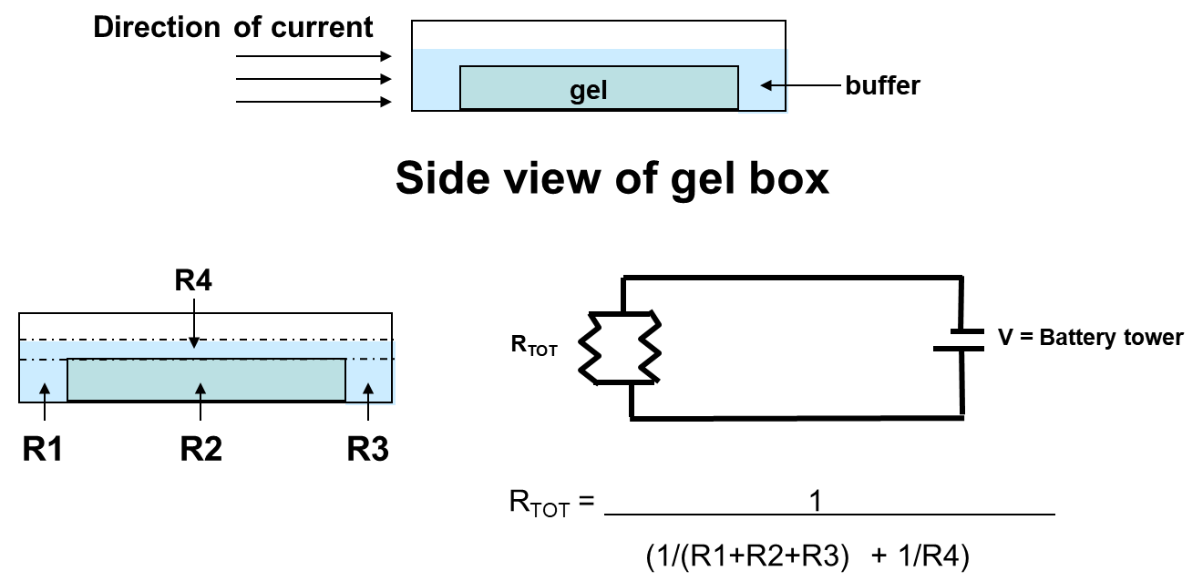
Fig 1: Physics of electrophoresis
Electrophoresis involves charged particle passing through a liquid under an applied potential difference. An electrophoresis cell, fitted with two electrodes, enables dispersion of particles of target mixture. When a potential is applied across the electrodes, the electronegative colloid particles migrate to the oppositely charged electrode. The resistance of the cell can be calculated by measuring the voltage and current and the impact of buffer on-resistance can then be equated to find the molecular size of particles.
Components of electrophoresis:
Electrophoresis is usually performed by setting gels and casting them in tubes, slabs, or on a flatbed. In many electrophoresis units, the gel is mounted between two buffer chambers containing separate electrodes, so that the only electrical connection between the two chambers is through the gel.
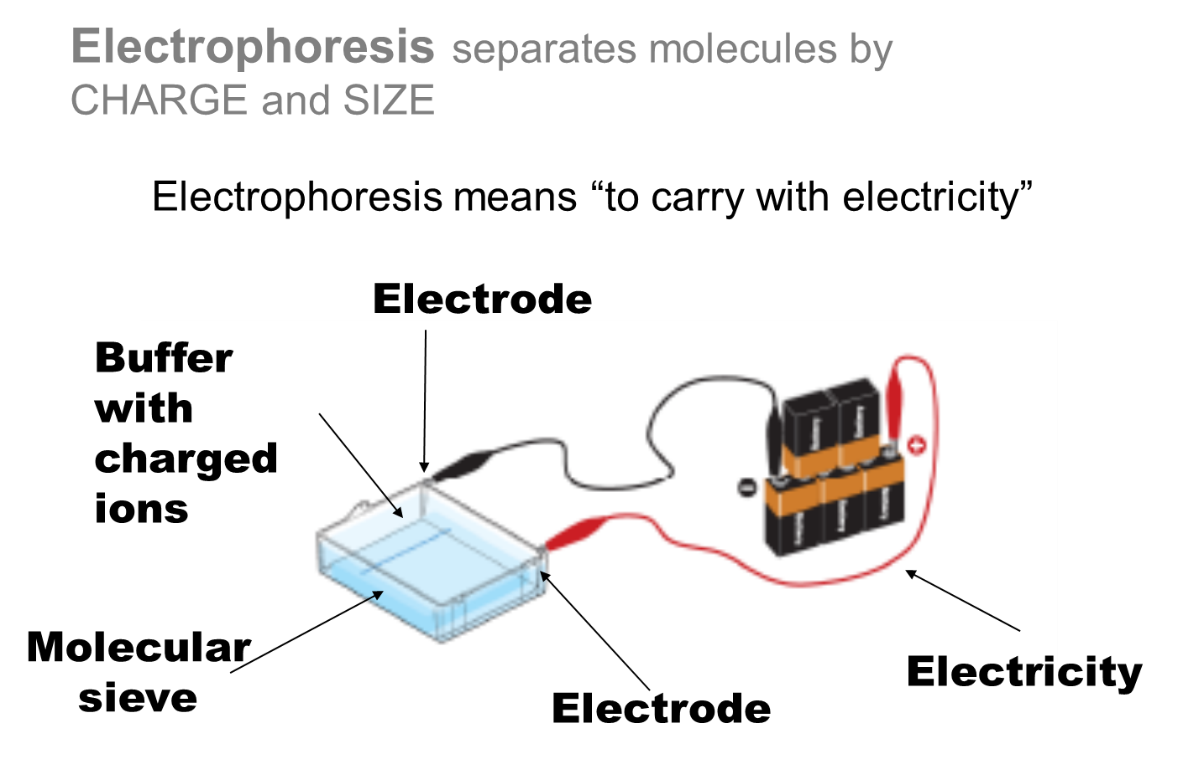
Fig 2: Electrophoresis separates molecules by charge and size.
The buffer
Buffer with charged ions must buffer the target mixture and not change pH significantly with the increase in temperature. It must be capable of carrying a charge and must be capable of solubilizing a gel matrix molecule. It must maintain an orderly distribution of the electric field and not interfere with future reactions. Also, it must not heat up too much during a run.
The electrodes
Electrodes must be capable of carrying charge with minimal chemical transformations occurring while immersed in a salty solution and be relatively chemically inert in a salty solution. They must be malleable enough to mold to desired dimensions. They should also be reusable for fairly permanent fixation into an instrument (ie do not want to have to replace regularly).
The Molecular Sieve
The molecular sieve must be capable of separating molecules via size and be easily moldable. It should not chemically interact with the molecules being separated. It should have a high enough melting point that electrophoretic runs will not melt it. If polymeric should be of molecular purity such that there is no batch to batch differences.