Phenols are organic hydrocarbon compounds with a hydroxyl group directly attached to an aromatic hydrocarbon compound like Benzene Ring. Phenols are also termed as Phenolic compounds. The molecular the formula of phenols is C6H5O6. The synthesis of Phenols is Natural as well as artificial.
Acidic nature of phenol
The acidity of phenol is one of the important characteristics exhibited by the phenol compounds. The phenol compound is formed by the hydroxyl group attached to the aromatic benzene group. The OH (hydroxyl) group is attached to the sp2 hybridized carbon of the benzene ring. Due to sp2 hybridization, the electronegativity is quite high in the carbon. Thus, the electron density at the Oxygen atom of the hydroxyl group decreases subsequently.
The phenol can be further converted to phenoxide ion by reaction with bases like NaOH or KOH. As these bases are stronger they attack the OH group of phenol and remove the H atom. Thus, the ionic bonded phenoxide ion is formed. Thus, these reactions of phenols with metals like sodium and potassium show the acidic nature of phenols.
As the OH group of phenols is attached to the benzene carbon, it becomes weak in electron density. Thus, the acidity of phenol is more than compared to alcohols. As a result, the dissociation of phenol’s H atom is more than that of alcohols in preferred solvents. Also, the acidity of phenols is more than that of water. Hence, more phenol molecules give H atom than water in proper solvents.
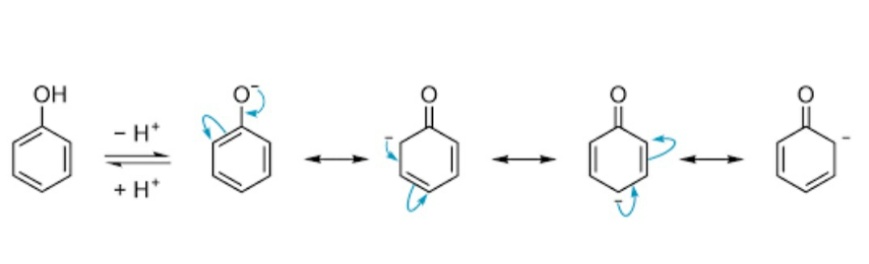
The acidic nature of phenols can be further understood by the resonance of the negative charge in the phenols. The resonance is observed in the phenoxide ion this generated due to reaction with a base. The negative charge of is resonated at the ortho, meta and para positions. The acidity of phenols could be explained by the stability of the negative charge generated in the phenoxide ions. Further by the effect of resonance, if these groups are attached at the ortho or para positions, the acidity of phenols could be further increased. This is because the negative charge generated in the phenoxide ion is majorly stabilized at the ortho and para positions in the benzene ring by resonance.
The acidity of phenols could be increased by the attaching of the electron withdrawing groups. As it increases the electronegativity of the phenol. On the other hand, the acidity of the phenol is further decreased due to the attachment of the electron donating groups to the compound. This is because of the formation of phenoxide ion becomes difficult if the electron donating group is attached.