Ozonolysis is the method of oxidatively cleaving the alkenes by using the ozone which is a reactive molecule of oxygen. This process allows the double or triple bonds of the carbon-carbon to be replaced by the double bonds with the oxygen. It is an organic reaction, where unsaturated bonds of the alkenes are cleaved with the ozone. This process is also used for cleaving the unsaturated bonds of azo compounds and the alkynes. The outcome of the ozonolysis reaction strongly depends on the workup conditions and the types of multiple bonds to be oxidized.
Mainly, this reaction is used for the identification of the structure of unknown alkenes by breaking them into small pieces so that they can be easily identified. Ozonolysis is a natural phenomenon as well and it breaks the repeated units which are used in the polymers and the rubber. On the industrial scale, pelargonic acids and the azelaic acid are produced by the process of ozonolysis.
The most interesting fact in this alkene reaction is that it does not only breaks the carbon-carbon π bond but also breaks the carbon-carbon σ bond. This reaction is known as oxidative cleavage as along with oxidation it results in the cleavage of the bonds and ozonolysis is the most important and prominent example of the oxidative cleavage. As earlier, it is known fact that the ozone absorbs much of the ultraviolet radiation and protect the life from damage. In the traffic-clogged cities, ozone causes the breathing problem. It is a powerful oxidant and it is used for the cleavage of carbon-carbon multiple bonds.
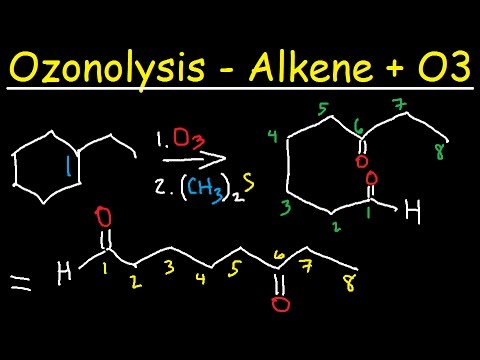
In the process of the ozonolysis, the ozone which is present in the gaseous state is passed through the desired solution of the alkene in either dichloromethane or ethanol. Ozonide is obtained as the first intermediate product, which is further reduced to the carbonyl products. As a result, the double bond of carbon-carbon is broken and is replaced by the double bond of carbon-oxygen instead.
In the very first step in the mechanism of ozonolysis, there is an initial electrophilic addition of the ozone to the carbon-carbon double bond which results in the production of the molozonide intermediate. This molozonide molecule is unstable so it further continues with the reaction and is broken apart to make the carbonyl and carbonyl oxide molecule. Then the carbonyl oxide and carbonyl are rearranged and remake the ozonide intermediate. Then a reductive workup can be performed for converting the ozonide molecule to the desired carbonyl products. The second step in the process of ozonolysis is called workup and there are two different types of workup. The reductive workup is useful as it helps to preserve all other aspects of the molecule and saves the double bond.
Earlier ozonolysis has been employed for the structural determination of the unknown molecules. The analysis of fragments can be used to deduce the original structure of the molecule by stitching together all of the fragments. Especially, this was significantly important for the unsaturated molecules known as the terpenes.