Orbitals in the same subshell are called degenerate orbitals and in these orbitals, electrons are filled in such a way that the pairing of electrons occurs only after each of the degenerate orbitals (same energy) occupies one electron. This is the Hund’s Rule of maximum multiplicity which also emphasize that the unpaired electrons present in orbitals of the same subshell should have parallel spins. Electron that are identical in charge tend to repel each other which can be minimized when these electrons are moved further apart by occupying different degenerate orbitals.
For example, carbon has 6 electrons and its electronic configuration is 1s22s22p2. The first two subshells are fully filled however 2p subshell contains two electrons and they cannot treat either orbital differently since they belong to the same energy level. Hence, the electrons occupy two different orbitals with the same spin direction as portrayed in Figure 1 (Huheey, Keiter, Keiter, & Medhi, 2006)
.
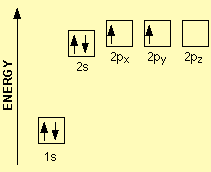
Figure 1: Electron configuration of carbon.
An electron shall not pair with another in a half-filled orbital before having single occupancy in all of them. Therefore, at ground state when two electrons come in contact with each other they behave like magnets. First, they try to repel and move further away from each other and then pair up with opposite spins once they cannot move further at the same energy level. Since there are 1, 3, 5 and 7 orbitals in s, p, d and f subshell respectively, the pairing of electrons start with the entry of 2nd, 4th, 6th and 8th electron respectively (Singh, 2011). Moreover, half-filled and fully filled orbitals acquire more stability due to the symmetrical distribution of electrons.
Also, electrons in the outer shells do not the full force of positive charge of the nucleus due to the presence of inner shell electrons. Because of this screening effect the net positive nuclear charge decreases with an increase in angular quantum number or ‘l’.
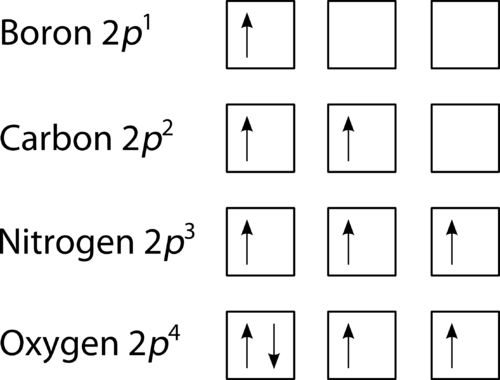
Figure 2: The orbital diagrams visually reconstruct the electronic configuration by presenting each orbital separately and spins on the electrons. This is determined by first defining the subshell (s, p, d or f) and then drawing each electron within the box.
As shown in Figure 2, the electrons enter each orbital first before pairing up. Notice that in Nitrogen (Z=7), Hund’s rule depicts that the lowest energy arrangement is maintained with three unpaired electrons. And with Oxygen (Z=8) one electron must be pairs with one 2p orbital which gives two another unpaired electrons. Unpaired electrons are paramagnetic and the paired electrons are diamagnetic as they are repelled from a magnetic field. Table 1 shows the capacity of the first four principal energy levels.
| Orbitals and electrons in the first four principal energy levels | ||||
| Principal energy level (n) | Type of subshell | Number of orbitals (n2) | Orbitals per type | Maximum number of electrons (2n2) |
| 1 | s | 1 | 1 | 2 |
| 2 | s, p | 4 | 1, 3 | 8 |
| 3 | s, p, d | 9 | 1, 3, 5 | 18 |
| 4 | s, p, d, f | 16 | 1, 3, 5, 7 | 32 |