Preparation of sodium chloride:
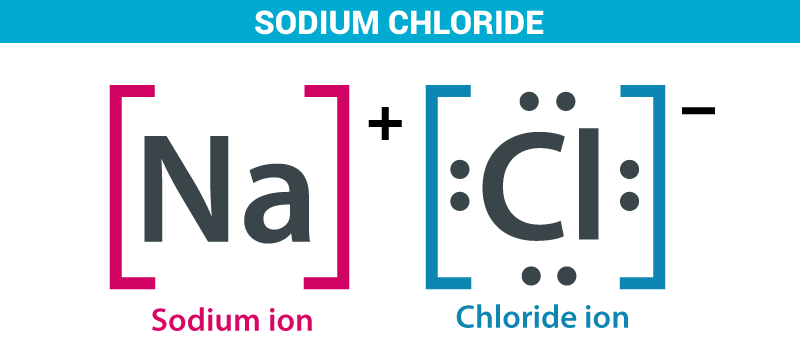
As we all know sea water is the major source of sodium chloride. Sodium chloride is an ionic solution between sodium and chloride. Seawater contains about 2.7-2.9% of sodium chloride. It is mainly responsible for the salinity of the water. It is also known as common salt.
- In tropical countries like India, it is generally obtained by evaporation of sea water. In India, around 50 lakh tons of salt are produced annually by solar evaporation. The evaporation of seawater usually gives crude sodium chloride which generally contains impurities of calcium sulphate, sodium sulphate, calcium chloride, and magnesium chloride. This considered being impurities because they are deliquescent and absorbs moisture easily from the atmosphere. Now for the purification of this crude salt, it is dissolved in the minimum amount of water and filtered to remove insoluble impurities. Then HCl gas is passed through the solution to get the saturated solution of salt. Due to the common ion effect between HCl and NaCl crystals of pure sodium chloride separate out. The salts of calcium and magnesium chloride remain in the solution only because they are more soluble in water. Hence, Pure sodium chloride is obtained.
- Brine is also treated by multiple-effect vacuum evaporator. The device contains three or maybe more closed metal drums with conical shaped base. Brine is first processed chemically to remove calcium and magnesium impurities. Then it is filled in the bottom of the metal drums. The brine in the first cylinder passes through the tubes which are heated by vapor. Then the brine boils because of heating and its steam comes in the next drum, and so on the process goes on. In each cylinder pressure of inside is dropped because of the condensation of steam permitting the brine to boil at a much lower temperature. Salt is then removed from the bottom of the drums as the dense slurry. It is then filtered to eliminate excess brine, dried, and distributed through the screens to sort the particles by size.
Properties of sodium chloride:
- Sodium chloride is a white crystalline solid.
- Sodium chloride doesn’t possess the odor but has a characteristic taste.
- Sodium chloride has a melting point of 1081 K (801 degree Celsius) and boiling point 1713 K (1413 degree Celsius).
- Sodium chloride is soluble in water and its solubility is 36g per 100g of water at 273 K but partially soluble or insoluble in other liquids.
- Pure sodium chloride is considered to be non-hygroscopic but due to impurities of calcium and magnesium chloride, it behaves as hygroscopic.
- In the aqueous state, NaCl acts as a good conductor of electricity due to the free movement of the ions.
Uses of sodium chloride:
- Sodium chloride is used as table salt or common salt for domestic purposes, an essential constituent of our food.
- It is used in the manufacture of sodium carbonate, sodium hydroxide, etc.
- Sodium chloride is used in a freezing mixture.
- It is also used for salting out soap.
- It is used in the tanning and textile industries.