Compounds are made up of different elements and each has own chemical identity, so, the use of chemical formulas is considered as most succinct way to represent the elemental makeup.
Empirical Formula
It is formula which gives simples ratio of whole numbers of atoms in any given compound. It gives the composition of elements of a substance in the terms of percentage. By determination of empirical formula and molecular mass, molecular formula can be easily determined that is just a multiple of whole number of empirical formula. The chemical formula obtained from composition analysis is empirical formula later on it can be used as a key step to find out the molecular formula by knowing the molecular weight of given compound.
Steps for Determination of An Empirical Formula
Following steps are involved to determine an empirical formula.
- First of all, consider the number of grams of all elements stated in the problem. If it is given in terms of percentage, then assume that total mass is equal to 100 grams.
Mass of each element = Given percentage
- In second step convert mass of every element to the moles by using molar mass that is given in the periodic table.
- Then divide the value of each mole by smallest number of moles that has been calculated earlier.
- In last step round the value to nearest whole number. It is then represented by subscripts in empirical formula and it is the mole ratio of the elements.
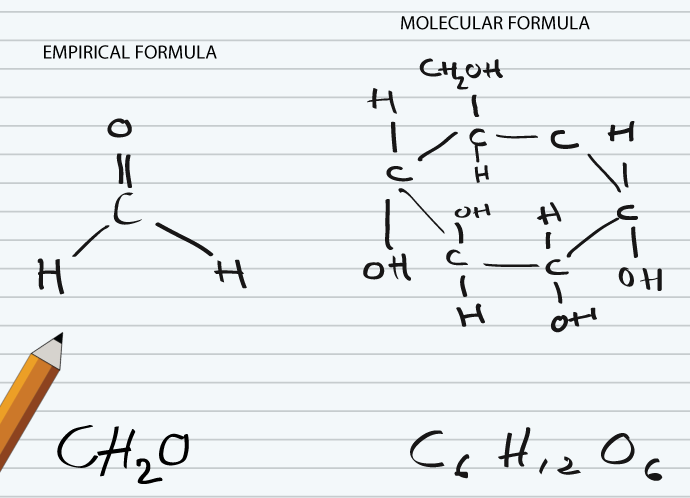
Molecular Formula
Molecular formula tells about number of atoms of each element in a compound. It cannot be determined by combustion analysis. It is always integer multiple of empirical formula as it is obtained by integer multiplication of the subscripts of empirical formula. By knowing empirical formula, its is easy way to find out molecular formula. For example, glucose has empirical formula CH2O, while its molecular formula is C6H12O6. So, empirical formula can be converted to molecular formula by multiplication with a whole number. Here are some other examples, the empirical formula of dichlorine hexoxide is ClO3, while its molecular formula is Cl2O6. For hydrogen peroxide empirical formula is HO and molecular formula is H2O2.
Molecular formula does not give any information about atoms arrangement. So, molecular formula can describe various chemical structures.
Difference Between Empirical Formula and Molecular Formula
Empirical formula of any given compound only gives the ratio of elements which are present in any compound. It does not provide any information about the actual number of atoms present in that compound. Even the empirical formula can be same for two compounds. So, due to this reason, empirical formula is not considered as true formula of compound.
While, on the other hand, molecular formula gives actual number of the atoms of every element present in the compound and is considered as true formula. Sometimes, it may happen that empirical formula and molecular formulas are same or molecular formula can be multiple of empirical formula. Empirical formula is not enough for elemental analysis of any unknown sample as it brings ambiguity, so, determination of molecular formula is necessary accompanying by other confirmation tests.