The electron was discovered by J. J. Thomson in 1897, while he was studying the properties of the cathode ray. In 1906 he was awarded a noble prize for the discovery of elemental particle electron. In 1937 he won the noble prize for providing wavelike properties of the electron.
In the late of the 19th century, J. J. Thomson started experimenting with the cathode ray tubes. Most of the air has been evacuated from cathode ray tubes and these are sealed glass tubes. There are two electrodes at one end of the tube, one of the electrodes is cathode that has a negative charge and the other one is anode that has a positive charge. A current of high voltage is applied across these electrodes and as a result, a beam of particles tends to flow from cathode to the anode. This ray can be detected by using a painting material phosphor onto the far end of the tube beyond the anode. When the phosphors is impacted by cathode rays, phosphors emits the light or it sparks.
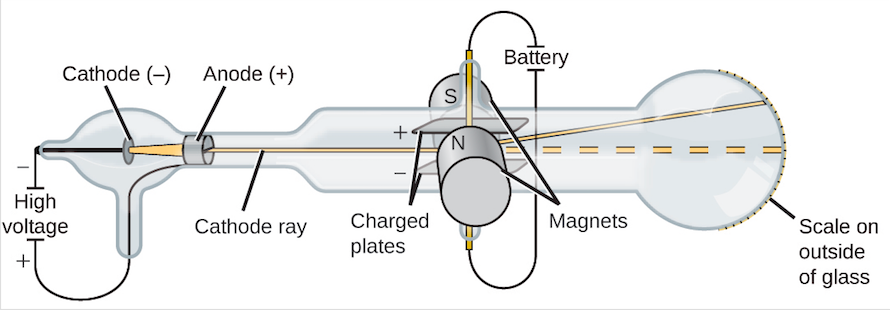
For testing the properties of the emitted particle Thomson placed electric plates around cathode ray and these plates were oppositely charged. Cathode ray showed deflection away from the negatively charged electric plates and towards the positive charged electric plate. It was an indication that particles in cathode rays were negatively charged.
Then on either side of the tube, Thomson placed two magnets and observed that cathode ray was also deflected by the magnetic field. Then Thomson determined mass to charge ratio by using the magnitude of deflections of the particles of cathode ray from a magnetic and electric field which led him to the fascinating discovery as the mass of each particle was too much smaller than any other known atom. He measured and compared this ration with the number of atoms. He repeated the experiments several times, by using different metals for electrode material and results were surprisingly amazing as, despite the origin, the properties of cathode rays remained constant. By using this evidence, he made some conclusions which are as under.
- Cathode ray is composed of the negatively charged particles.
- This particle should exist as part of the atom, as the mass of each of the particles was only ∼ 1/2000 of the mass of hydrogen atom.
- These are subatomic particles and can be found within the atoms of all of the elements.
At first, this discovery was controversial, but later on, it was gradually accepted by the scientists. Eventually, the particles emitting from cathode rays were given a unique name called electrons. Virtually, an electron was 1000 times lighter than the smallest atom, so it was the reason why cathode rays can easily penetrate the metal sheets.
Based on this discovery of the electron, a part of Dalton’s atomic theory was disapproved as his theory stated that atoms cannot be subdivided. This existence of electrons revealed that the atom is a complex structure and 2000 years old concept to consider the atom as a homogenous particle was wrong. Later on, many atomic models were presented by scientists to completely study the existence of electrons.