p-Block Elements: Group 15
Phosphine, halides PCl3, PCl5 and oxoacids
Phosphine is produced by the reacting calcium phosphide with water or dilutes HCl.
Ca3P2 + 6H2O3Ca(OH)2 + 2PH3
Ca3P2 + 6HCl 3CaCl2 + 2PH3
At laboratory scale, it is made by heating white phosphorus with concentrated NaOH solution in an inert atmosphere of carbon dioxide and produces sodium hypophosphite.
P4 +3NaOH + 3H2O PH3 + 3NaH2 PO2
In the pure state, phosphine is non-inflammable but becomes inflammable in the presence of P2H4 or P4 vapours. To purify it from the impurities, it is absorbed in HI to form phosphoniumiodide (PH4I) which on treating with KOH gives off phosphine.
PH4 I + KOH KI + H2O + PH3
Properties:-
1. 3CuSO4 + 2PH3 Cu3P2 + 3H2SO4
2. 3HgCl2 + 2PH3 Hg3P2 + 6HCl
3. Phosphine is weakly basic and like ammonia, gives phosphonium compounds with acids
e.g., PH3 + HBr PH4Br
Halides
The general formulas are PX3 and PX5
- PF3 is found in gaseous state
- PCl3 is found in liquid state( bp = 74oC)
- PBr3 is found in liquid state (bp = 175oC)
- PI3 is an unstable red solid (mp = 61oC)
PX3 + 3 H2O(l) H3PO3 (aq) + 3 HX(aq)
- PCl5 is an ionic saltof : PCl6– and PCl4+ ions.
- PBr5 is an ionic salt of PBr4+ and Br – ions.
- The PX5 compounds react with water to form Phosphoric acid.
PX5 + 4 H2O(l) H3PO4 (aq) + 5 HX(aq)
PCl3 or P4O6 are hydrolysed in water to form ox0acids of phosphorous.
| PCl3 | PCl5 |
| Colourless oily liquid | Yellowish white powder |
| Preparation
P4 + 6Cl2 4PCl3 P4 + 8SOCl2 4PCl3 +4SO2 +2S2Cl2 |
Preparation
P4 +10 Cl2 4PCl5 P4 + 10SO2Cl2 4PCl5 +10SO2 |
| Is hydrolysed in the presence of moisture
PCl3 +3H2O H3PO3 + 3HCl |
PCl5 + H2O POCl3 + 2 HCl
POCl3 + 3H2O H3PO4 +3HCl |
| Pyramidal shape, sp3 hybridisation | TBP geometry, sp3 d hybridisation |
| With acetic acid
3CH3COOH +PCl3 CH3CoCl + H3PO3 |
With acetic acid
3CH3COOH +PCl5 CH3CoCl + POCl + HCl |
| With alcohol
3C2H5OH + PCl3 C2H5Cl + H3PO3 |
With alcohol
C2H5OH + PCl5 C2H5Cl + POCl3 + HCl |
Phosphorous oxoacids
H3PO3 has only two acidic H atoms; the third is bonded to the central P and does not dissociate.
Orthophosphoric acid, H3PO4 has three acidic H atoms. It is a weak acid, but in the strong base, all three H+ is lost to give the phosphate anion.
Is the oldest known phosphorus compounds. It is a syrupy liquid made by direct reaction of ground phosphate rock with sulfuric acid. The pure acid is a colourless crystalline solid. Stable and has no oxidising properties below 350-400 °C. It will attack quartz. Hydrogen bonding persists in the concentrated solution and is responsible for the syrupy behaviour.
The acids in +3 oxidation state of phosphorus tend to disproportionate to higher and lower oxidation states. For example, ortho phosphorous acid (or phosphorous acid) on heating disproportionate to give orthophosphoric acid (or phosphoric acid) and phosphine.
4H3PO3 3H3PO4 + PH3
The acids which contain P–H bond has strong reducing properties. Thus, hypophosphorous acid is a good reducing agent. It contains two P–H bonds which can reduce AgNO3 to metallic silver.
4 AgNO3 + 2H2O + H3PO24Ag + 4HNO3 + H3PO4
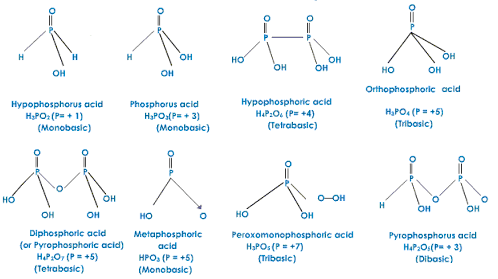
Fig 1: Oxoacids of phosphorus with their oxidation states.