In the early 1800s, the chemists were aware of only 30 elements, but with the development of nuclear physics, scientists are now able to identify more and more elements present on the earth. Based on the IUPAC standards, the current periodic table contains 118 elements. Among these elements, 92 are natural elements and the remaining are synthetic. Therefore, it has become increasingly difficult for a scientist to classify these elements into different groups and express their properties more significantly. Therefore, a classification of the element is necessary to separate like elements from unlike and create a systematic study of these elements. Otherwise, it becomes impossible to understand the physical and chemical properties of numerous elements at once. (Singh, 2011). Classification of elements also helps in understanding the type of compounds that can be formed using different elements, thereby correlating their properties efficiently.
The periodic table has certainly made the study of chemical and physical properties of elements simpler and organized. Using this table, scientists find it easy to refer the periodic table and its group designated to a certain element to see how the properties of elements in that group correlate and are able to predict the properties of other elements. Even though so many elements have already been discovered, new element keeps adding into the periodic table. Likewise, scientists can take help from the trends represented by elements in the periodic table and based on these characteristic properties identify new elements that have similar properties to the one that has ordered already been discovered. We can notice that some empty spaces have been left within the periodic table in order to allow more elements to be discovered in future without disturbing the periodicity trends of other elements.
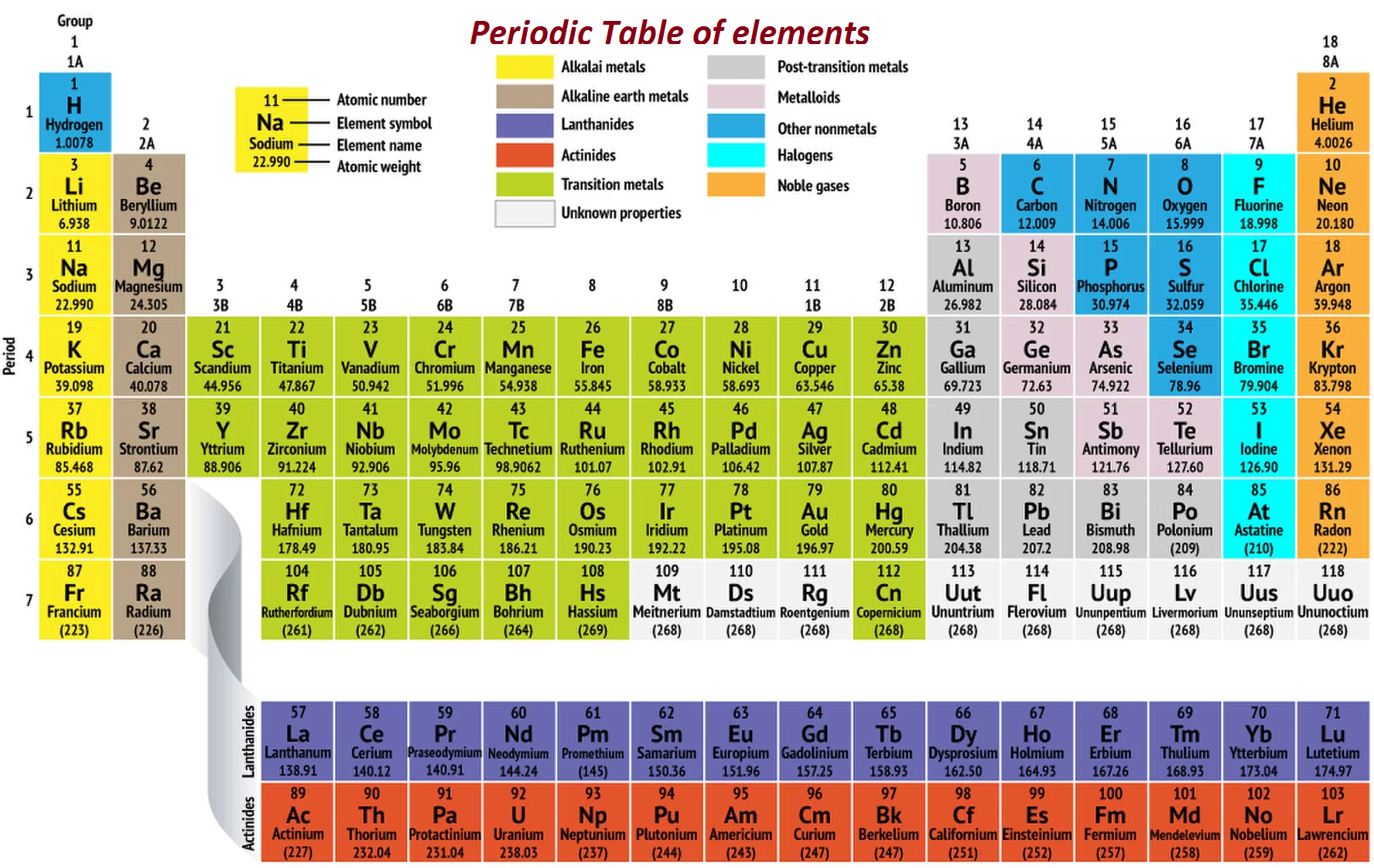
Figure 1: Different groups in the periodic table colour coded for ease of understanding (Huheey, Keiter, Keiter, & Medhi, 2006).
Earlier attempts to classify elements proposed that the elements should be divided into metals and nonmetals. Chemists defined that an element is a metal when it’s lustrous i.e. has a shiny surface and it is a good conductor of heat and electricity, it has ductile and malleable properties. It should also be solid at room temperature and has the ability to lose valence electrons. However, Mercury and Gallium are liquids at room temperature and Zinc is not malleable or ductile. On the other hand, an element is considered a nonmetal if it is not lustrous, is a bad conductor of heat and electricity, has no ductility or malleability and it has a tendency to gain one or more electron. A nonmetal can be a gas, liquid or a brittle solid at room temperature. However using this classification method, the chemist was not able to justify for the more active metals and nonmetals also known as metalloids. Examples of metalloids include Silicon Germanium Arsenic and Antimony.