It was a major challenge before the scientists at the end of the 19th century to reveal the structure of the atom as well as to explain its important properties. For this different atomic models were proposed. After Thomson’s model failed to explain certain experimental results, Rutherford, a British scientist conducted an experiment and based on the observations of this experiment gave Rutherford Atomic Model.
Rutherford’s Alpha Scattering Experiment
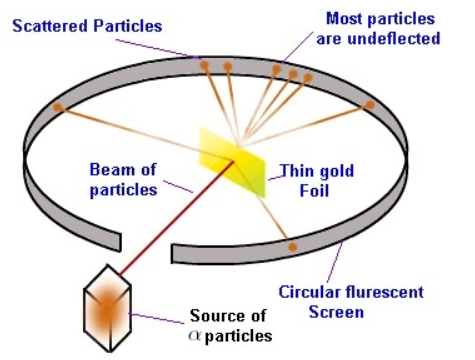
For his experiment a thin sheet of gold was bombarded with α-particles. He then studied the trajectory of these particles after their interaction with the gold foil.
He directed high energy streams of α-particles from a radioactive source at a thin sheet of gold (100 nm thickness). For studying the deflection caused to the α-particles, he placed a fluorescent zinc sulphide screen around the thin gold foil. Thomson’s atomic model was contradicted by the observations made by Rutherford.
Observations of Rutherford’s Alpha Scattering Experiment
Rutherford’s observation led him to conclude that:
- A major fraction of the α-particles bombarded by Rutherford towards the gold sheet passed through it without any deflection, and therefore most of the space in an atom is empty.
- Some of the α-particles were deflected by the gold sheet by very small angles, and hence it was concluded that the positive charge in an atom is not uniformly distributed. It actsually concentrated in a very small volume.
- Only a few of the α-particles had nearly 180o angle of deflection that is very few α-particles deflected backwards. So in comparison to the total volume of an atom the volume occupied by the positively charged particles in an atom is very small.
Rutherford’s Atomic Model
Based on the observations and conclusions of his experiment, Rutherford proposed the atomic structure of elements. According to the Rutherford atomic model:
- Most of the mass of an atom and the positively charged particles were concentrated in an extremely small volume. This region of the atom was called as a nucleus.
- His model proposed that the negatively charged electrons surround the nucleus of an atom. He also claimed that the electrons which surround the nucleus revolve around it with very high speed in circular paths. These circular paths were named as orbits by him.
- Nucleus being a densely concentrated mass of positively charged particles and electrons being negatively charged particles are held together by a strong electrostatic force of attraction.
Limitations of Rutherford Atomic Model
Rutherford atomic model also failed to explain certain things like Thomson’s Model although it was based on experimental observations.
- Rutherford model was not in accordance with Maxwell’s theory of electromagnetic radiations and it could not explain the stability of an atom. He proposed the electrons to be revolving around the nucleus in fixed paths called orbits. As per Maxwell’s theory, electromagnetic radiations are emitted by accelerated charged particles and hence an electron which revolves around the nucleus should emit electromagnetic radiation. This radiation from the electron would carry energy from the motion of the electron which would come at the cost of shrinking of orbits. Ultimately the electrons would collapse in the nucleus. If this were true, the atom should be highly unstable, and hence matter would not exist in the form that we know. But we know that atoms are quite stable.
- Rutherford model did not say anything about the arrangement of electrons in an atom which made his theory incomplete.
Although the early atomic models like Thomson’s model and Rutherford’s model were inaccurate and failed to explain certain experimental results, they were the base for future developments in the world of quantum mechanics.