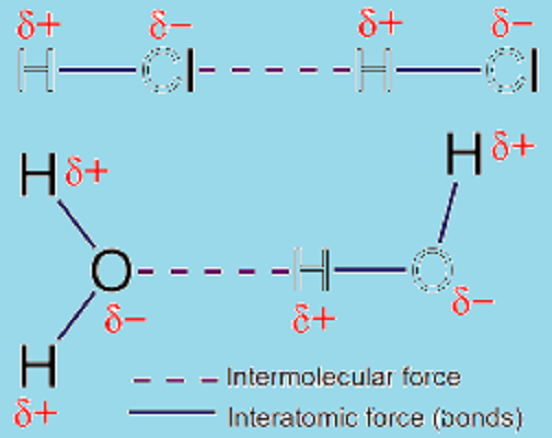
Intermolecular forces prevail along intermolecular interactions being forces of repulsion or attraction among neighboring particles that may be ions, molecules or atoms. These forces are comparatively weaker than the covalent and iconic bonds that are formed among atoms in a molecule. For instance, the covalent bonds that may prevail within Hydrogen Chloride (HCl) are supposed to be stronger than the bonds that it is likely to form with other neighboring external elements.
Intermolecular interactions are the ones that occur among one or more molecules are called intermolecular interactions; whereas the ones that may occur between different atoms of the same molecule are termed as intra molecular interactions. These interactions could occur among ions and molecules in prevalent states of matter.
These interactions would include even the weakest forces of dispersion that could not be explained well yet and the strongest long-distance electrical repulsions and attractions that would take place among ions. Intermolecular interactions are important since without that the condensed form of matter (solids or liquids) would not exist except in extremely low temperatures.
In the decreasing order of the strength of interactions, here are the different types of intermolecular interactions:
Ion-Ion Interactions
The concept for ion-ion interaction is probably the easiest to understand. While like charges repel, opposite charges are known to attract each other. While in the gaseous phase, Coulombic forces tend to operate over distances that are relatively longer. Certain oppositely charged particles that may flying in the vacuum, tend to attract each other. The forces between them eventually get stronger, and once they stick to one another a certain amount of energy would be essential to separate them.
This produces an ion pair that is an individual new particle which possesses a negatively charged and a positively charged area. These ion pairs continue to interact with individual ions so that the cluster continues to grow and falls out eventually of the gas phase in the form of a liquid or solid, based on the temperature.
Dipole Moment
Consider the first ion pair that was formed when the positive and negative ions came together. If the electronegative for considerable elements are different sufficiently then the charges of the ion pair may not change appropriately. The blue ones would carry the full electron charge while the red ones would carry full positive charge.
An ionic bond would be formed by the attraction among these opposite charges. Electronegativity does not bring up much difference however; there would be considerable sharing of electrons among the two atoms. Whether two atoms come together or two atoms, results are likely to remain the same.
Dispersion forces
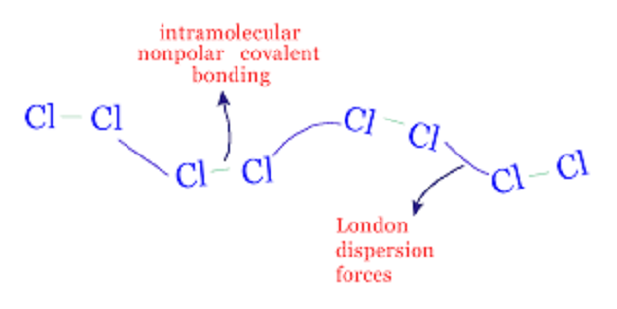
The interactions between dipoles, ions and induced dipoles are likely to account for molecular deviations through ideal gas behavior that may be in the vapor state or the condensed form that would be in the solid or liquid forms. In other words, stronger interactions would allow the liquid and solid state of the elements to persist high temperatures. Non-polar molecules however show similar behavior which indicates some type of intermolecular interaction although these cannot be ascribed as the simple electrical attractions. These interactions are termed as dispersion forces.
The electrical forces tend to operate when the molecules are several molecular diameters away and get stronger as the ions or molecules begin to approach one another. These forces are still weak unless the ions or molecules have almost embraced one another.