A carbocation is an ion that is associated with the positively charged carbon atom. The simplest examples of carbocations are methanium and the vinyl cations. Occasionally, some carbocations can bear more than one positively charged carbon atom e.g. ethylene dictation. Earlier carbocations were known as the carbonium ions. Carbocations are mainly classified in the two main categories according to the coordination number of the charged carbon.
Carbocations can be considered as protonated alkanes. These are much electrons poor. It is easily destabilized by an electron withdrawing group. Some carbocations can have two or more than two positive charges on the same or the different carbon atoms.
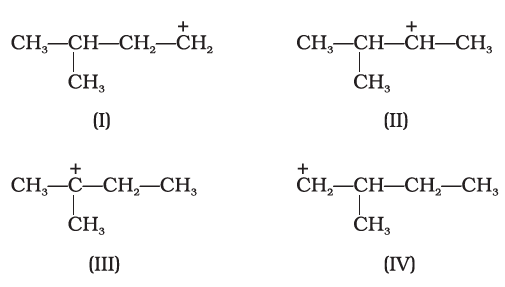
Carbocations are known since 1891. In carbocation the charged carbon atom is sextet and it has six electrons in the outer valence instead of eight electrons so it does not ensure the maximum stability according to the octet rule. Thus, carbocations are the reactive species and are in a continuous struggle to fill the valance electrons to regain the neutral state. The reactivity of carbocations closely resembles that of sp2 hybridization having the atrigonal planar molecular geometry. Often carbocations are targeted by the nucleophilic attack by the nucleophiles such as halogen ions or hydroxide ions.
Typically, carbocations go under the rearrangement reactions and they are converted from the less stable structures to the more stable structures with a rate constant that is more than the 109 per second. Carbocations can be stabilized by the resonance by a double bond of carbon-carbon next to the ionized carbon. The carbocations in which the + carbon is adjacent to the other atom of carbon having double or triple bond are extra stable due to the overlapping of the empty p orbitals of the carbocation. Due to this overlapping charge is shared between the multiple atoms, causing the delocalization of the charge and therefore, carbocation is stabilized.
The relative stability of the carbocations can be explained by the hyper-conjunction. Some of the carbocations e.g. 2-norbornyl cation exhibit the more or less of the three centers two electron bonding symmetry and they are called as non-classical carbocations. It happens due to the delocalization of the bonds. Nowadays a variety of carbocations are known that have non-classical structures.
Other than the classical and non-classical carbocations a third form is also known to exist that are pyramidal carbocations. In this structure single carbon atom hovers the four or five-sided polygon making the pyramid. The ion having the four-sided pyramid contains the charge of +1 and five-sided pyramid has the charge of +2. The energy difference between the classical and non-classical carbocations is very small and little activation energy is involved in the transition between the classical and non-classical carbocations.
Specific carbocations such as Cyclopropylcarbinyl cations are studied by using the NMR. The NMR spectrum of dimethyl derivative shows the presence of two non-equivalent signals and indicates that its molecular conformation is not perpendicular but it is bisected with empty p orbital and to the cyclopropyl ring system in the same plane.