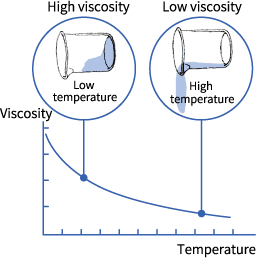
Viscosity is defined as the resistance to the flow of liquid. Usually, for liquids, the greater is the IMF (intermolecular forces), the higher is the viscosity.
The molecules’ shape and temperature are two of the prime factors which affect viscosity. High temperature has a close similarity with the high average kinetic energy and with quickly moving molecules. This will result in a lower viscosity. Viscosity is also affected by the shape as molecules having several twists or branches will be difficult to “glide-by” each other than smaller “round” molecules.
An illustration of this phenomenon is visualizing a contest between two fluids down the windshield. Which windshield will roll down faster, either water or honey, what’s your opinion? Clearly, from the experience, you’ll expect honey will efficiently speed up than the water. In fact, it also reveals that honey consists of higher viscosity level than water.
Introduction
Viscosity is the phenomenon which is not only related to resistance to the flow of liquid but even related to resistance to the flow of gas, shape change, or movement. Unlike viscosity, there is fluidity which measures the effortlessness of flow, whereas fluids like honey or motor oil which are “slow-moving” and high in viscosity, are referred to as viscous.
A question may arise in your mind that what exactly is going between one type of liquid which is flowing faster and another one which is more resistant to flow like the comparison between water and honey. The reason is fluid’s part moves, it pressurizes other neighboring liquid parts to move alongside, generating internal friction between molecules that eventually results in a decreased flow rate.
In fact, it’s even important to keep in mind that gases and liquids’ viscosity are affected by the temperature but on contrary ways which means that during the heating process, liquid’s viscosity goes down drastically, on the other hand, the flow of gases is more in a sluggish manner. What’s the reason behind this case? Well, as the temperature advances, molecules’ average speed in the liquid increases too and as a consequence, they tend to spend comparatively lesser time with their “neighbors”.
Thus, as temperature accelerates, the average rate of intermolecular forces goes down, and molecules have the capability of interaction without having the condition of “weighed down” by each other. However, gas’s viscosity tends to rise higher with the increase in temperature as the intermolecular collisions’ frequency increases at high temperatures.
Measuring Viscosity
There are several ways to measure viscosity. One of the simplest and basic methods is to allow a sphere, like a metal ball, falling through the fluid and the time of falling of the metal ball: as slow as the sphere ball drops, the more is the viscosity which measured.
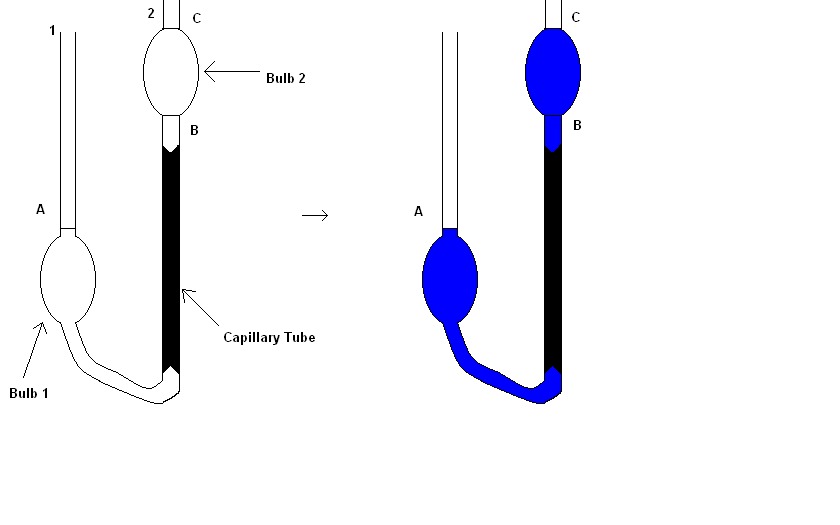
One more enhanced design to measure viscosity known as Ostwald Viscometer, which is very much accurate than releasing a metal ball from a height. An Ostwald Viscometer has 1 capillary tube and 2 reservoir bulbs.
The viscometer remains filled with the liquid until the liquid reaches A mark with the help of pipette to precisely measure the quantity of a required liquid. After that, the viscometer is then inserted into a water bath, which balances the temperature of the test liquid.
As mentioned earlier, “equilibration” plays a significant role in maintaining a constant temperature as viscosity should not be affected otherwise. Then the liquid is drawn through the second side of U-tube by suction use and in the end, the flow is given by time between flow C and B marks.
The calculation of viscosity is done by using Equation
η=Kt (1)
Where,
K = value of a liquid whose density and viscosity is known like water.
Once K’s value is known, the viscosity can find out by measuring the time limit the test fluid flows in between two qualified marks.