Periodic trends are the repetition of similar atomic properties after regular intervals and they are caused by elements with similar electronic configuration aligning in the same group or period. Atomic properties constitute the physical characteristics of an atom. These properties include atomic radius, ionic radius, ionization energy, electronegativity, electron affinity, valance etc.
The atomic radius is a distance from the centre of the nucleus to the outermost shell of the electrons in the atom of any element. Since an electron cloud surrounds an atom, it does not have sharp boundaries. Hence, the determination of atomic size cannot be precise. An estimate of atomic radii can be made by knowing the distance between atoms in a combined state. For example, the covalent radius of an element is measured by taking half of the distance between two atoms bound together by a single bond whereas a metallic radius can be calculated by taking half the internuclear distance separating the metal cores in the metallic crystal. Likewise, depending on whether the element is metallic or non-metallic, the atomic radius can be referred to both covalent and metallic radius. Atomic radii can also be measured by X-ray and other spectroscopic methods.
Alternatively, the noble gases are monatomic and their non-bonded radii values are very large. Therefore, their radius is measured using Van der Waal’s method. Van der Waal’s radius is measured by taking half of the internuclear separation between two atoms of the same element adjacent to each other in their solid state.
i.e Van Der Waals radius > Metallic radius > Covalent radius
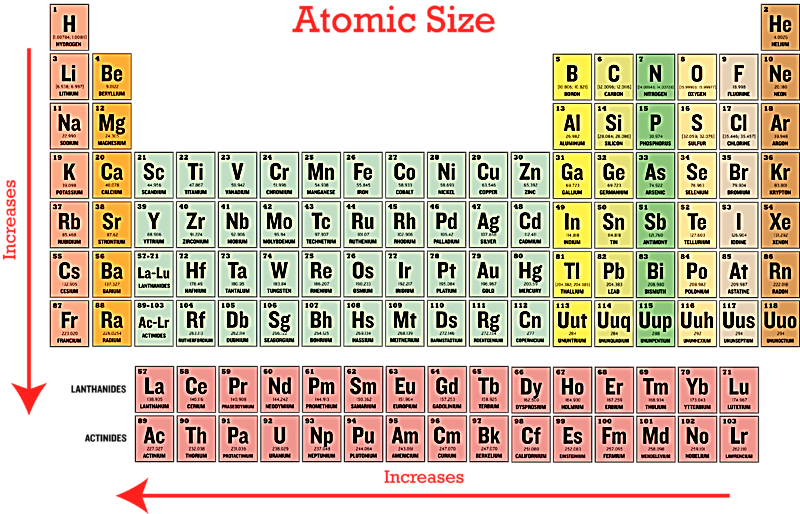
In the periodic table, the atomic radius of the element decreases from left to right in a period. As we move from left to right in the periodic table, the positive charge on the nucleus or the effective nuclear charge increases making the attraction of the outer electron to increase. This makes the electron closer to the nucleus which causes the atomic size to decrease.
In a group, the atomic radius of the element increases from top to bottom of the group. Down the group, the number of shells in the atom increases which increases its size. For halogens and alkali metals as we go down the group, the principal quantum number ‘n’ increases and the valence electrons move further away from the nucleus. The inner energy levels are filled with electrons that serve as a shielding agent between the nucleus and the valence electrons causing the size of an atom to increase. When the nucleus pulls strongly on the valence electrons, the valence shell is pulled in closer to the nucleus. When the attraction is weakened by shielding, the valence shell cannot be pulled closer. Although the valence electrons participate in shielding, electrons in the same energy level do not shield each other as effectively as the core electrons do. Hence, the amount of shielding mainly depends on the number of electrons between the nucleus and the valence electrons.