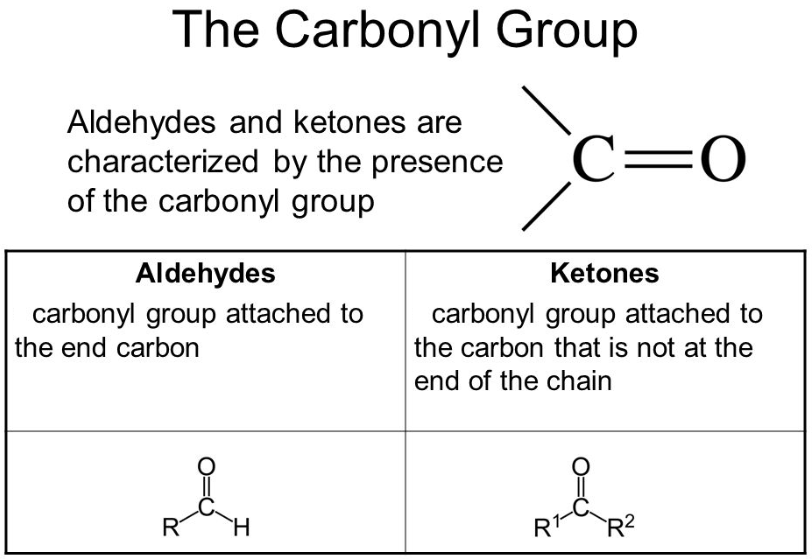
Chemically, the carbonyl group is the organic functional group that is composed of the carbon atom that is having a double bond with the oxygen atom. Ketones and aldehydes are the simplest carbonyl groups and usually, they are attached to another carbon compound. In any aromatic compound, their structure can be found and they have a major contribution to the taste and the smell of the aromatic compounds. The situation where carbon makes the double bond with the oxygen is considered as the carbonyl group entity and the members of the group are known as the carbonyl compounds. Generally, the carbon and oxygen of the carbonyl group are planar and sp2 hybridized.
In terms of reactivity, the double bonds of the carbonyl groups are very different. The double bond between the carbon-carbon is less reactive due to the presence of the double bond between the carbon and oxygen, so, the electronegativity is attributed to the oxygen and the two lone pairs of electrons that oxygen is having. One of the lone pairs is located in the 2s and other is located in the 2p orbital. The properties of the carbonyl groups are directly related to the geometric positioning and the electronic structure. Due to the electronegativity of the oxygen pi bond is also polarized and it allows the single-bonded substituent to be the electron-withdrawing. The length of the double bond of the carbonyl group is about 1.2 angstroms and it has the strength of about 176-179 kcal/mol. The length of the carbonyl group can be correlated with its polarity i.e. if the bond is longer then its polarity is lower. For example, in the acetaldehyde, the bond length of the C=O is larger than that of formaldehyde.
Due to the presence of two lone pairs of the electrons that are hanging around the oxygen, carbon is less electronegative than the oxygen. So, the carbon is electrophilic that is partially positive and the oxygen is nucleophilic as it is partially negative. A lowercase delta is used to denote the polarizability and the use of negative or positive superscript depends upon the conditions. The boiling points of the aldehydes and ketones are also affected due to the polarizability of the carbonyl group and they are higher than the other hydrocarbons of the same amount. The larger carbonyl compounds are less soluble in the water. If the carbonyl compounds have more than six carbons, it becomes insoluble in water.