p-Block Elements: Group 15
Nitrogen: Preparation, properties and uses
Nitrogen is a very abundant element that is colourless, odourless and tasteless. It was first discovered by A Scottish physician, Danial Rutherfords in 1772. It occurs in all life forms with aminoacids, chlorophyll and nuclide acids. The human body contains 3% nitrogen. Nitrogen happens to be the 7th most abundant element in the universe.
N2is chemically unreactive because of strong triple bond holding N’s together. Nitrogen occurs as dinitrogen. N2 (bp 77.3 K).78% of the atmosphere is N2. N14/N15 has a ratio of 272.N15 compounds are used in tracer studies.The N≡N triple bond is responsible for the inert behaviour of N2. It forms strong pπ-pπ multiple bonds due to its small size. N2 has a high bond strength and a short internuclear distance (1.094Å) and it can form upto 4 bonds. P forms infinite layer structures with only single bonds or P4 molecules.
In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
NH4CI(aq) + NaNO2 (aq) → N2 (g) + 2 H2O (l) + NaCl (aq)
It can be obtained by the thermal decomposition of ammonium dichromate.
(NH4)2Cr2O7 (Heat) → N2 (g) + 4 H2O (l) + Cr2O3 (s)
Very pure nitrogen can be obtained by the thermal decomposition of sodium or barium azide.
Ba(N3)2 → Ba + 3 N2
N2 is prepared by liquefaction and fractionation of air. This process is called cryogenic distillation. First, the air is compressed and cooled down followed by purification, cooling and then fractional distillation to store various gases at their respective boiling point. The boiling point of nitrogen is 77K. This process produces very high purity nitrogen at a lesser cost than other processes.
It can also be produced by reduction of air with carbon or oxidizing ammonia.
Air (4 N2 + O2)+C → 4 N2 + CO2
NH3 +3O2 → 2 N2 +6H2O
2 NH3 + 3 Cl2 → N2 + 6 HCl
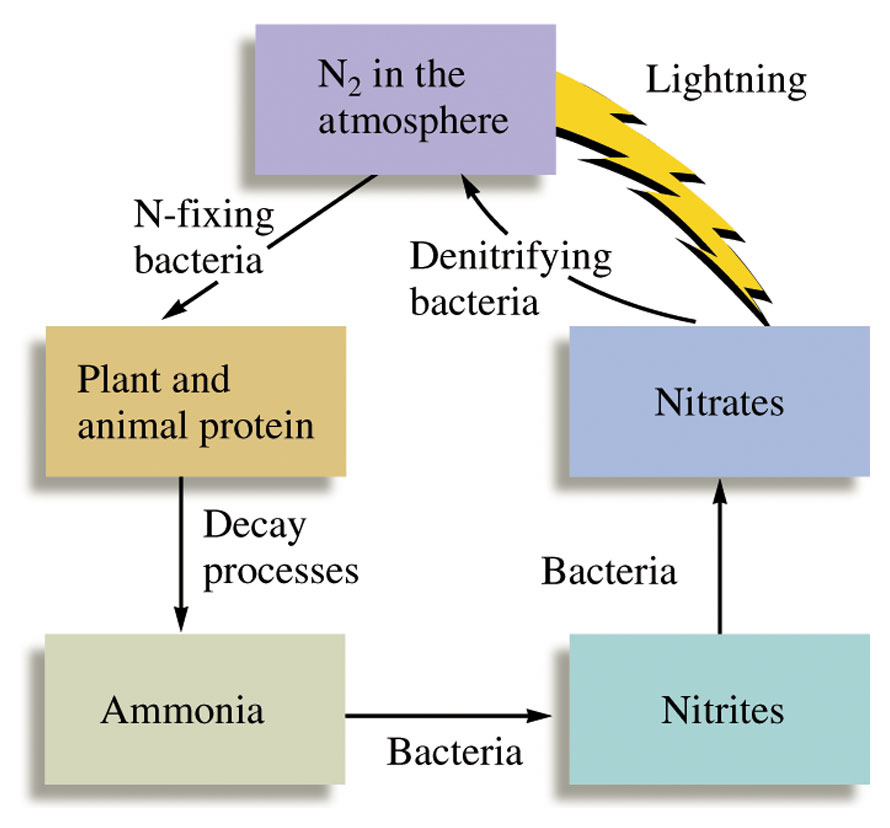
Fig 1: Nitrogen fixation allows organism to fix atmospheric nitrogen into biologically useful compounds.
Nitrogen is otherwise an inert gas but when mixed with hydrogen at 773K and 2-9 Atm, it produces ammonia. It also reacts with oxygen at 2273-3273 K in an electric arc to produce oxides of nitrogen. Highly reactive metals like magnesium, calcium and aluminium burn with nitrogen to form respective nitrides in an exothermic reaction.
The biggest commercial use for elemental nitrogen gas is for the formation of ammonia in the Haber process. The ammonia produced from the Haber process is mostly utilised in making fertilizers such as urea. Nitrogen is also used in chemical reactions where the inert environment is required, eg. In the iron and steel industry. Liquid nitrogen is also used as a refrigerant and for cryosurgery. Food packed with nitrogen increases its shelf life and help slow down bacterial growth. Liquid nitrogen is also used as a coolant in some MRI devices and other temperature control systems. Nitrous oxide is also known as laughing gas that is used in dentistry and other topical surgical procedure for anaesthetic purposes.