Sodium carbonate is also known as washing soda. Its chemical formula is $Na_{2}CO_{3}.10H_{2}O$.
Preparation of sodium carbonate: sodium carbonate is mainly prepared commercially by Solvay process or ammonia-soda process. A Simple process of sodium carbonate preparation is shown below:
$2NaCl+CaCO_{3} \to Na_{2}CO_{3}+ CaCl_{2}$
The reaction shown above is reversible and creates other problems in the system. Hence, other chemicals are required to get the desired product. Therefore, nowadays it is prepared on the basis of a principle described below:
Principle of the process: when carbon dioxide gas is passed through a brine solution saturated with ammonia, it gives sodium bicarbonate. The precipitated sodium bicarbonate is filtered and dried and is ignited to give the final product sodium carbonate as shown below:
$2NH_{3}+ H_{2}O+CO_{2} \to (NH_{4})_{2}CO_{3} Ammonium carbonate$
$(NH_{4})_{2}CO_{3}+H_{2}O+ CO_{2} \to 2NH_{4}HCO_{3} Ammonium bicarbonate$
$NH_{4}HCO_{3}+ NaCl \to NaHCO_{3}+ NH_{4}Cl sodium bicarbonate (ppt.)$
$2NaHCO_{3}\to Na_{2}CO_{3}+ CO_{2}+ H_{2}O Sodium carbonate$
Raw materials for the process are:
- Sodium chloride (NaCl)
- Limestone for carbon dioxide $(CaCO_{3})$
- Ammonia gas $(NH_{3})$
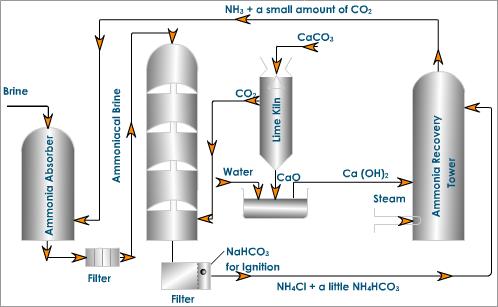
Let me tell you a bit about this process of manufacturing sodium carbonate. The plant is shown in the figure below. The plant involves the following steps:
- Ammonia absorber: About 30% of brine is pumped into ammonia absorber. A mixture of ammonia and a small amount of carbon dioxide is bubbled through the brine solution so that it gets saturated with ammonia.
- Carbonation tower: The ammoniated brine solution that we got in the first step is introduced from the top of a tower known as the carbonation tower. In this tower, carbon dioxide is admitted from the base which rises through the small perforations of the tower. The brine solution trickles down and meets the upgoing vapors of carbon dioxide and forms insoluble sodium bicarbonate.
- Filtration: In this step, the milky solution from the carbonation tower is filtered with the help of a rotary vacuum pump to separate the sodium bicarbonate.
- Calcination of sodium bicarbonate: sodium bicarbonate that we got from the carbonation tower is heated in the kiln to form sodium carbonate.
- Ammonia recovery tower: The filtration from the step-3 is mixed with calcium hydroxide and heated with steam in ammonia recovery tower with calcium hydroxide. Ammonia along with a small amount of carbon dioxide is pumped to the top of the brine saturation tower.
- Lime kiln: The carbon dioxide required in the carbonation tower is prepared by heating limestone to about 1300 K in a lime kiln.
Properties of sodium carbonate:
- Sodium carbonate is white crystalline solid exists in nature as a decahydrate $a_{2}CO_{3}.10H_{2}O$. It is generally known as washing soda.
- Sodium carbonate is readily soluble in water.
- The Action of heat on sodium carbonate: on heating Sodium Decahydrate loses the water of crystallization to form Monohydrate $Na_{2}CO_{3}.H_{2}O$. Above 373 K more heating transforms sodium monohydrate into completely anhydrous and white powder called soda ash.
$Na_{2}CO_{3}.10H_{2}O \to Na_{2}CO_{3}.H_{2}O+9H_{2}O $ Sodium monohydrate$Na_{2}CO_{3}.H_{2}O \to Na_{2}CO_{3}+ H_{2}O $ Soda ash
- Hydrolysis: sodium carbonate gets hydrolyzed by water to form an alkaline solution. $CO_{3}^{- }+ H_{2}O \to HCO_{3}^{-}+ OH^{-} $
- The Action of acids on sodium carbonate: sodium carbonate reacts with dilute mineral acids evolving carbon dioxide gas as shown below:
$Na_{2}CO_{3}+ 2HCl \to 2NaCl+ $ $H_{2}O+ CO_{2}$ - Reaction with milk of lime: sodium carbonate reacts with hot milk of lime forming sodium hydroxide. $Ca(OH)_{2}+ Na_{2}CO_{3} \to CaCO_{3}+ 2NaOH$