We are all familiar with the matter. The definition of Matter is anything that has mass and volume (takes up space). For most common objects that we deal with every day, it is fairly simple to demonstrate that they have mass and takes up space. Everything that is around us including the pen, book, pencil, buildings, bridges, and atomic particles, all are composed of matter. Even our DNA, the air, the molecules inside our bodies, everything is matter. They all have mass and occupy space.
We know that matter comprises of particles. These particles are atoms and molecules. In this section, we will cover the nature of the matter. The matter only moves from one phase to another by the physical means. One can create a physical change if energy is added which means the temperature is increased or energy is taken away stating something is frozen. Based on its physical state, we can divide the nature of matter into three major categories states viz. solid, liquid and gas.
Figure
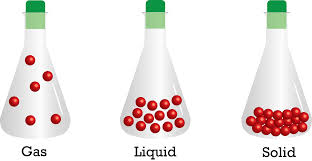
In solids, these particles are held very close to each other in an orderly fashion and there is not much freedom of movement. The solids have definite volume and definite shape. For example – Ceramic Bowls which are made from soft clay and is heated up initially and later slowly cooled. The clay becomes hard as the water is removed and chemical bonds are changed inside the clay.
In liquids, the particles are close to each other but they can move around. They have a definite volume but no definite shape and they take the shape of the container. The examples of liquid are – Oceans, Lakes, and rivers.
However, in gases, the particles are far apart as compared to those present in solid or liquid states and their movement is easy and fast. It neither has definite volume nor definite shape. They occupy the space completely. For example – Balloons which isn’t a gas, however, the helium (gas) inside the balloon is gas. It is a noble gas which has very low atomic mass and when in a gaseous state, it is lighter than the air.
Because of such arrangement of particles, different states of matter exhibit the following characteristics:
- Solids have definite volume and definite shape.
- Liquids have definite volume but not the definite shape. They take the shape of the container in which they are placed.
- Gases have neither definite volume nor definite shape. They completely occupy the container in which they are placed.
These three states of matter are inter-convertible by changing the conditions of temperature and pressure.
Solid ——————— Liquid ———————— Gas
On heating a solid, it changes to the state of liquid. On further heating the liquid, it changes into gas or the vapor state. If reversed, a gas when cooled, it liquefies to liquid and on further cooling the liquid it freezes in solids.
At the macroscopic or bulk level, matter can be classified as mixtures or pure substances. These can be further sub-divided as shown in Figure 2.
Figure
Many of the substances present around you are mixtures. For example, sugar solution in water, air, tea etc., are all mixtures. A mixture contains two or more substances present in it (in any ratio) which are called its components. A mixture may be homogeneous or heterogeneous. In a homogeneous mixture, the components completely mix with each other and its composition is uniform throughout. Sugar solution and air are thus, the examples of homogeneous mixtures. In contrast to this, in heterogeneous mixtures, the composition is not uniform throughout and sometimes the different components can be observed. For example, the mixtures of salt and sugar, grains, and pulses along with some dirt (often stone) pieces, are heterogeneous mixtures. You can think of many more examples of mixtures that you come across in daily life. It is worthwhile to mention here that the components of a mixture can be separated by using physical methods such as simple hand-picking, filtration, crystallization, distillation, etc. Pure substances have characteristics different from the mixtures. They have fixed composition, whereas mixtures may contain the components in any ratio and their composition is variable. Copper, silver, gold, water, glucose are some examples of pure substances. Glucose contains carbon, hydrogen and oxygen in a fixed ratio and thus, like all other pure substances has a fixed composition. Also, the constituents of pure substances cannot be separated by simple physical methods. Pure substances can be further classified into elements and compounds. An element consists of only one type of particle. These particles may be atoms or molecules. Sodium, copper, silver, hydrogen, oxygen, etc. are some examples of elements. They all contain atoms of one type. However, the atoms of different elements are different in nature. Some elements such as sodium or copper, contain single atoms held together as their constituent particles whereas, in some others, two or more atoms combine to give molecules of the element. Thus, hydrogen, nitrogen, and oxygen gases consist of molecules in which two atoms combine to give their respective molecules. This is illustrated in Figure 3.
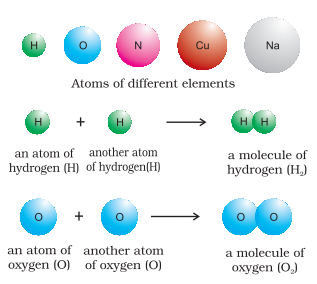
Figure 3
When two or more atoms of different elements combine, the molecule of a compound is obtained. The examples of some compounds are water, ammonia, carbon dioxide, sugar etc. The molecules of water and carbon dioxide are represented in Figure 4.
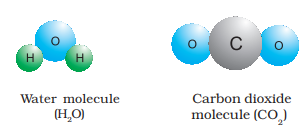
Figure
You have seen above that a water molecule comprises two hydrogen atoms and one oxygen atom. Similarly, a molecule of carbon dioxide contains two oxygen atoms combined with one carbon atom. Thus, the atoms of different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound. Also, the properties of a compound are different from those of its constituent elements. For example, hydrogen and oxygen are gases whereas the compound formed by their combination i.e., water is a liquid. It is interesting to note that hydrogen burns with a pop sound and oxygen is a supporter of combustion, but water is used as a fire extinguisher. Moreover, the constituents of a compound cannot be separated into simpler substances by physical methods. They can be separated by chemical methods.