p-Block Elements: Group 15
Oxides of nitrogen
Nitrogen forms six stable oxides at different oxidation states ranging from +1 to +5.Oxides with higher oxidation state are more acidic than the ones with lower oxidation state. Lightning stroke is a natural source of producing nitrogen oxide where nitrogen combines with oxygen in a lightning arc. Many of these oxides are also formed during the burning of fossil fuels and other high-temperature processes. These gases are a major pollutant from car exhausts that are removed using catalytic converters.
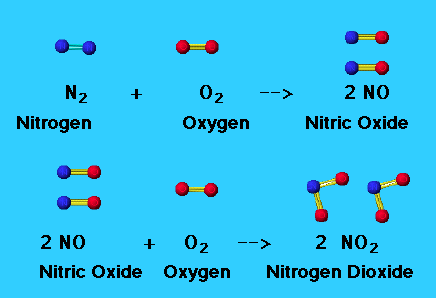
Fig 1: Formation of nitric oxide and nitrogen dioxide.
NOx is the main ingredient in the formation of ground-level ozone, triggering serious respiratory problems and acid rain. They react to form nitrate particles, acid aerosols, as well as NO2, which also contributes to nutrient overload that deteriorates water quality and increase global warming. ∆Hf for all six oxides is positive because of the great strength of the N≡N bond. Oxides are intermediates to HNO3 which is used as fertilizers.
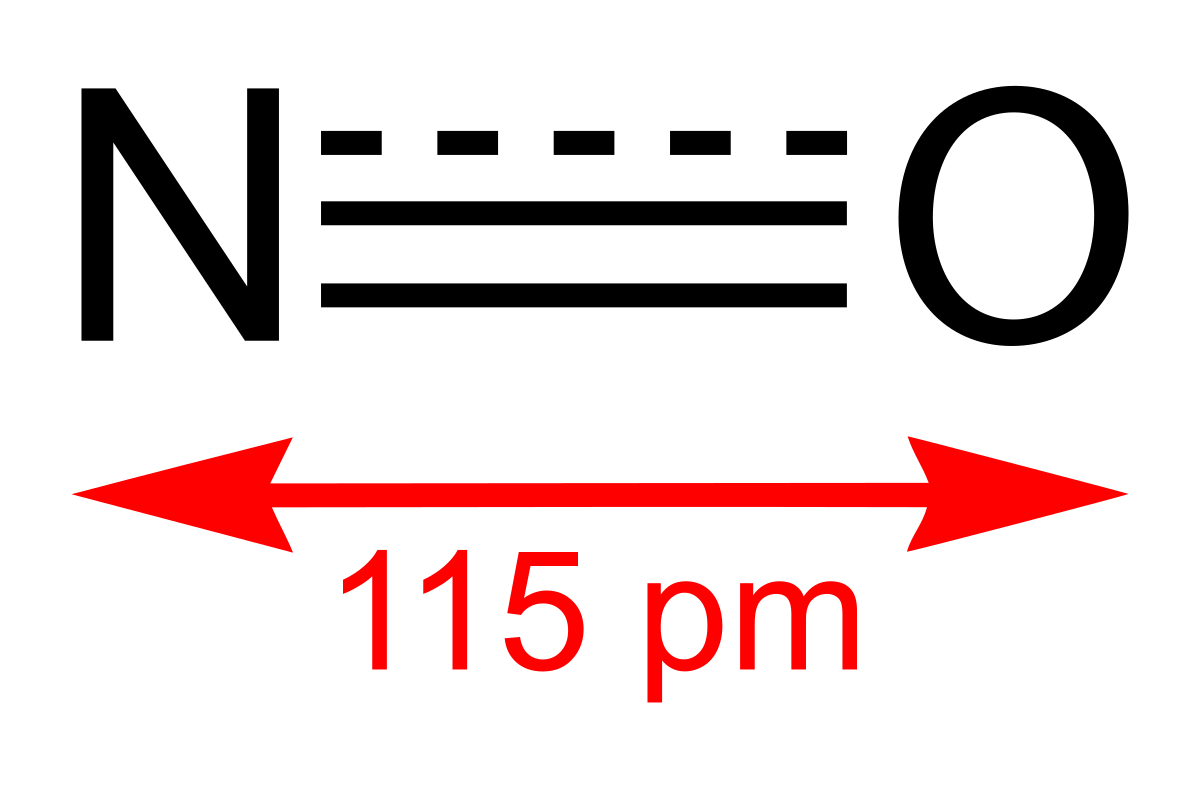
Nitric oxide, NO is a toxic, colourless and odourless gas that is insoluble in water. In water, it attains a blue colour. NO is produced by the oxidation of ammonia:
4NH3(g) + 5O2(g) → 4NO(g) +6H2O(g)
2NaNO3 (s) + 2FeSO4 (s) + 3H2SO4(l) Fe2(SO4)3 (s) + 2NaHSO4 (s)+2H2O(l) +2NO (g)
NO is converted to 2 other oxides by heating. This type of redox reaction is called disproportionation.
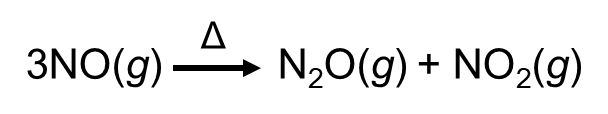
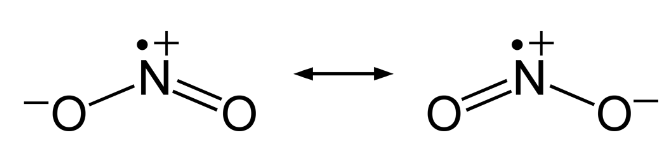
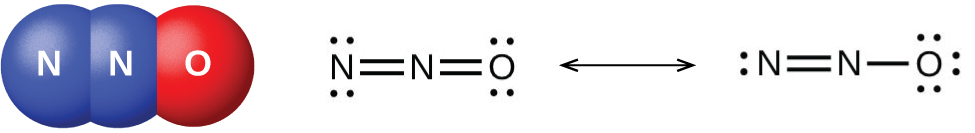
Nitrogen dioxide, NO2 is a component of toxic photochemical smog. Usually a dimer compound (N2O4) at low temperature. It has a distinct reddish-brown colour and it is moderately soluble in aqueous liquids. It is also a resonance hybrid that is a paramagnetic substance. NO, and NO2 react rapidly with other compounds and releasing harmful byproducts as well as depleting ozone. The NO and NO2in the atmosphere may never reach a high concentration but are rapidly changed into other harmful constituents that pollute the air. Due to the presence of unpaired oxygen electron, nitrogen dioxide dimerizes to form dinitrogen tetroxide.
2Pb(NO3)2 (673K) 4NO2 +2PbO +O2
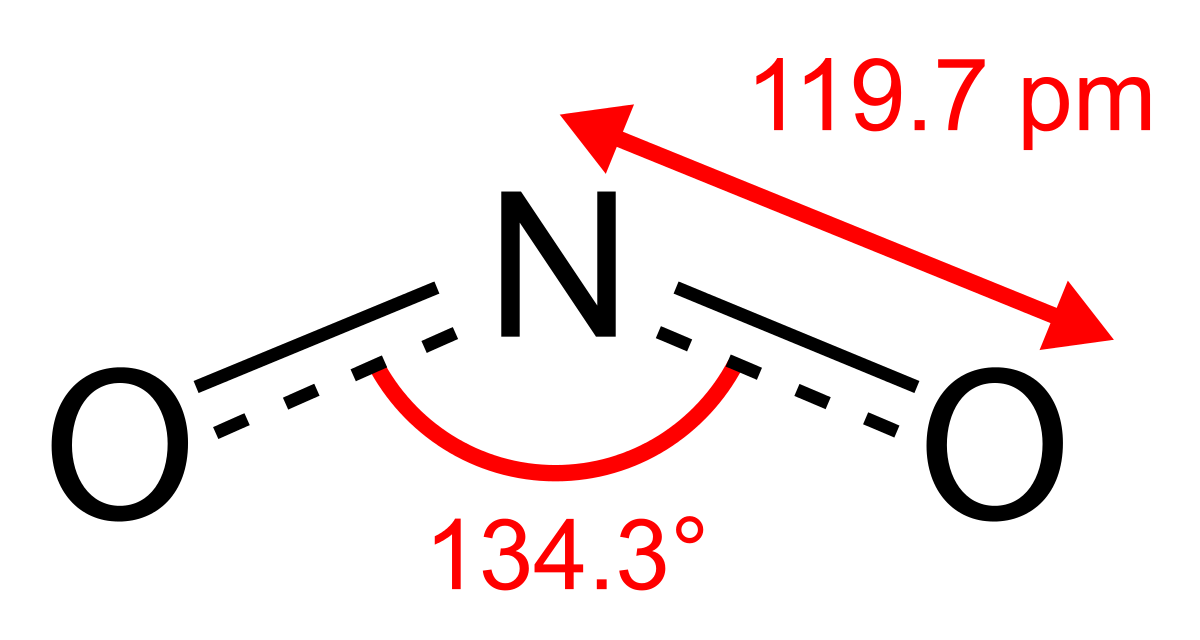
Dinitrogen monoxide, N2Oor nitrous oxide has a linear structure is relatively unreactive. It is inert towards Halogens, Alkali metals and Ozone at room temperature. It is called the laughing gas and is used as an anaesthetic
Dinitrogen trioxide, N2O3is an anhydride of nitrous acid. This gas is acidic in nature. It is planar in shape and is a resonance hybrid that is present in two forms. It is produced as a blue liquid when a mixture of dinitrogen tetroxide and nitric oxide are cooled together to 250 K.
2NO + N2O4 (250K) 2N2O3
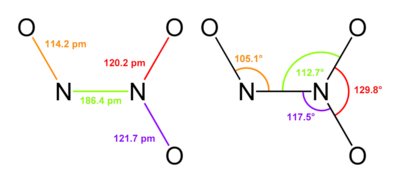
Dinitrogen Pentoxide (N2O5)
This compound is produced by dehydration of nitric acid with phosphorus pentoxide.
4HNO3+ P4O10 4HPO3 +2N2O5
It is crystalline deliquescent solid that is colourless and readily dissolve in water to form nitric acid. It is planar in shape and resonance hybrid which is acidic in nature. Dinitrogen pentoxidesublimes slightly above room temperature. It is an unstable and potentially dangerous oxidizer. It is used as a reagent when dissolved in chloroform for nitration process.
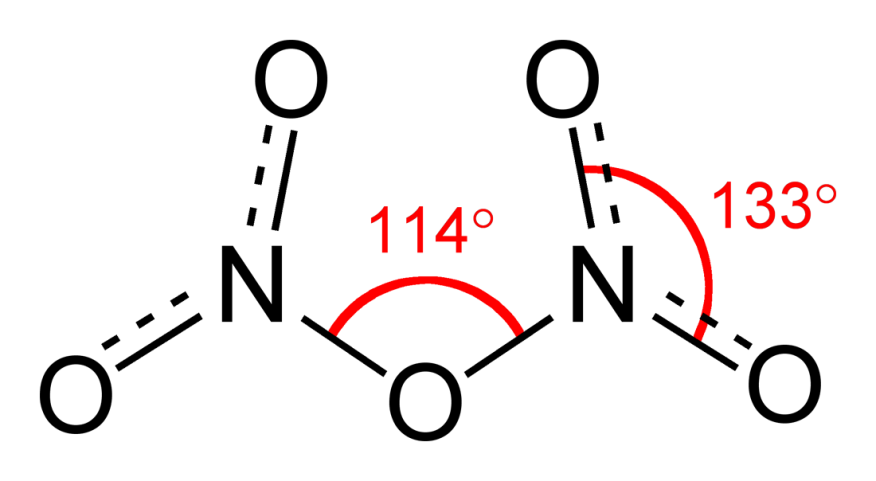
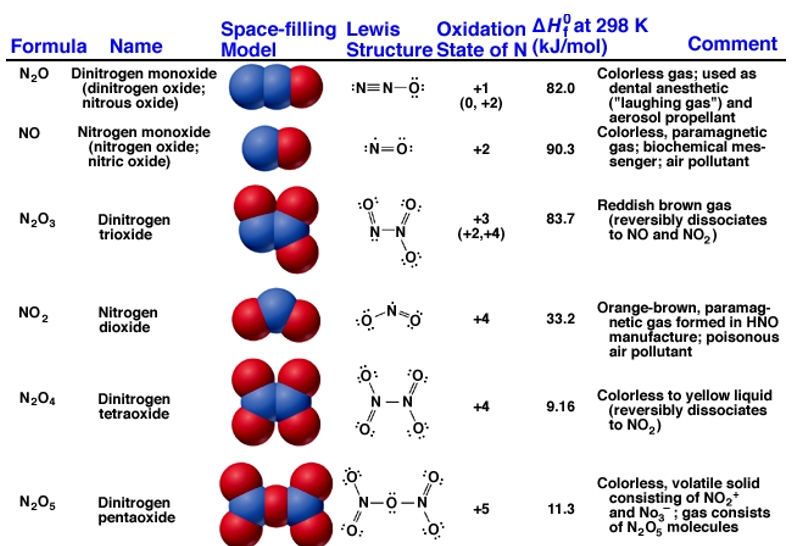
Fig 2: Structure and properties of nitrogen oxides.