Calcium oxide (CaO) also called as quicklime is an alkaline substance which has been in use since old age. Quicklime is believed to be one of the oldest chemical known to human race. It is also called as burnt lime. The used to prepare it is known as calcination. It involves thermal decomposition of the reactants at high temperatures but ensuring that the temperature is below melting point. The reaction is held in a rotary kiln. The products that are obtained from the reaction are calcium oxide and carbon dioxide. The reaction is exothermic and reversible in the forward direction.
CaCO3 → CaO +CO2
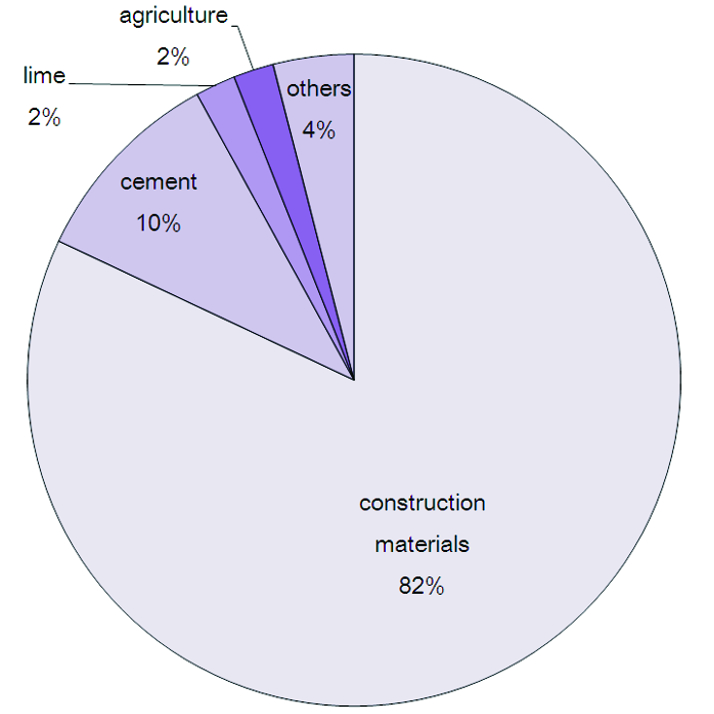
Calcium oxide is used in many industrial processes some of them are as follows:
Metallurgy
The most important industrial use of calcium oxide is Metallurgy, particularly in the production of steel. Calcium oxide reaction with sulphur, phosphorous, silica and other impurities present in the furnace from which steel is obtained. It also plays an important in the production of other metals such as aluminium and magnesium.
Pollution control devices
Smoke coming out from the chimneys of factories contains oxides of sulphur and nitrogen. It combines with water present in the atmosphere to form sulphuric acid and nitric acid respectively. To prevent this scrubbers are installed in smokestacks. It contains some chemicals that helps in neutralising the oxides of sulphur and nitrogen, one of the most important being calcium oxide.
Production of aerated concrete blocks
Hydrated lime along with quicklime can considerably increase the load carrying capacity of soil with contains clay. They react with finely divided silica and alumina to produce calcium silicates and aluminates, which possess cementing properties.
Petroleum Industry
Mixture of calcium oxide and phenolphthalein gives Water detection pastes. If this paste comes into contact with water in a fuel storage tank, the CaO will reacts with the water to form calcium hydroxide. The pH of Calcium hydroxide is high enough to turn the phenolphthalein into a vivid purplish-pink colour, thus indicating the presence of water.
Mining:
Compressed lime cartridges takes advantage of the exothermic properties of quicklime to break rock. A hole is drilled into the rock in the normal way and a cartridge of quicklime is placed within and tamped. A quantity of water is then injected into the cartridge and the resulting release of steam, together with the greater volume of the residual hydrated solid, breaks the rock apart.
Paper
Calcium oxide play an important role in paper making as it used to regenerate sodium hydroxide from sodium carbonate .
- Lime is used to neutralize acidic waste substances of industries which are ultimately natural water bodies without harming any aquatic beings.
Other uses of Calcium Oxide include:
- It is frequently used for medicinal purpose and insecticides.
- It is very efficacious in accelerating the decomposition of corpses.
- It is used as a reagent in laboratories for dehydration, precipitation, etc.
- It is an important ingredient in the manufacturing of caustic soda.
- It is also used for agriculture purposes especially for neutralising acidic soils.