SP2 hybridization is demonstrated by the carbonyl carbon and together its structure is coplanar. On the carbonyl’s electrophilic carbon atom, a nucleophile acts on the perpendicular direction to the orbital demonstration of sp2 hybridization of the carbon structure of the carbonyl. However, due to the attack of the carbonyl, the hybridization of the carbon is changed from sp2 to sp3 and thereby it yields the tetrahedral alkoxide intermediate. A proton will be taken by this reaction intermediate for the production of an electrically neutral compound. So, this reaction causes the addition of nucleophile and hydrogen to the carbon-oxygen double bond.
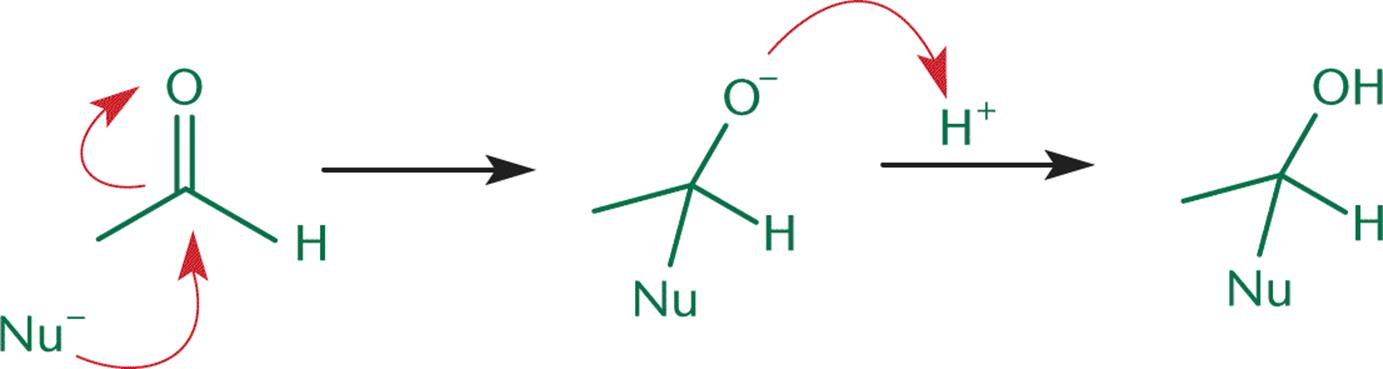
Both the ketones and aldehydes demonstrate the polar nature and as compare to the hydrocarbons they have higher boiling points. However, as compare to the alcohols, their boiling points are low. Many reactions involve the aldehydes and ketones and are sufficient for the various synthesis reactions. Nucleophilic addition to the carbonyl group is a major characteristic reaction of the ketones and aldehydes. Aldehydes readily undergo the nucleophilic addition reactions than the ketones, as aldehydes are more reactive.
A most favorable equilibrium constant is demonstrated by the aldehydes for the addition reactions due to the steric and electronic effect. While, for the ketones, two large substituents are present in their structure that causes the hindrance of the steric effect when nucleophile is approaching the carbonyl carbon. In the aldehydes one substituent is present and its steric hindrance is less when the nucleophile is approaching the carbonyl carbon. Moreover, if it is considered electronically, the reactivity of aldehydes is better demonstrated than the ketones. This is due to the reason that ketones have two alkyl groups due to which the electrophilicity of carbonyl carbon is decreased.
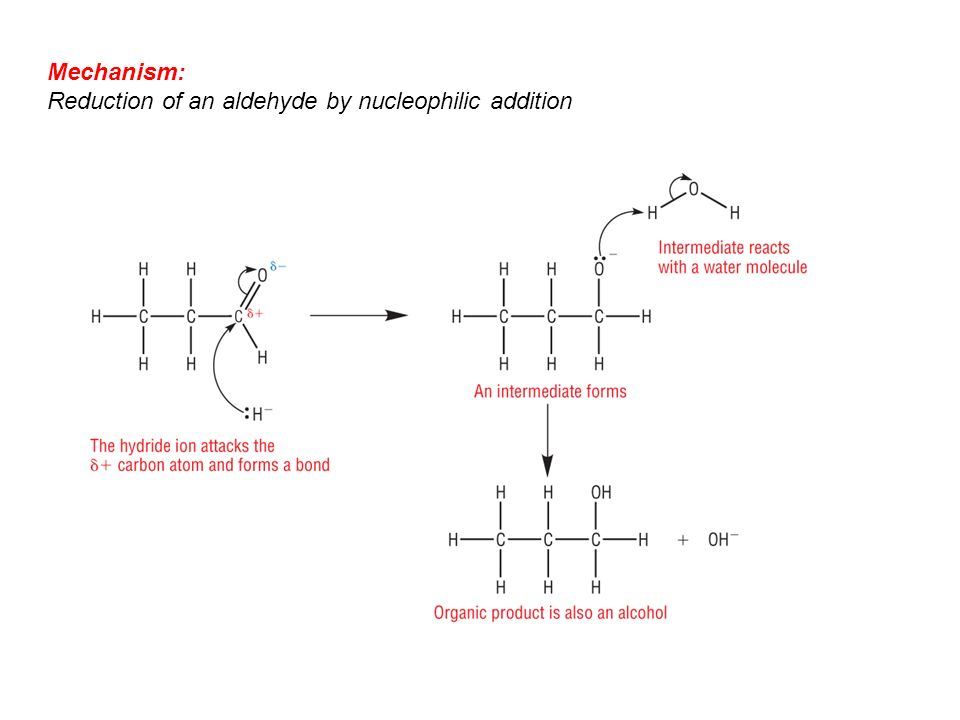
In this reaction, the step in which nucleophile attacks the carbonyl carbon is the most important one as it is the rate-determining step concerning the nucleophilic addition reaction which is base-catalyzed and to the nucleophilic addition reaction that is acid-catalyzed. In the case of the acid catalysis conditions, the protonation process occurs on to the carbonyl oxygen after the step of nucleophilic addition. Now due to the protonation, the carbocation character of the carbonyl structure is increased and causes it to be the more electrophilic. There are many nucleophilic addition reactions and the nucleophilic addition-elimination reactions such as the addition of hydrogen cyanide, the addition of sodium hydrogen sulfite, the addition of alcohols, the addition of Grignard reagent, and the addition of ammonia and its derivatives.