p-Block Elements: Group 16
Oxidation states
The elements of the oxygen family, group 16 appear in a number of oxidation states. The most common oxidation state is -2 because the general electron configuration is ns2np4. Chalcogens assume other oxidations state also including +2, +4 and +6. The stability of -2 oxidation state reduces as the metallic nature of the elements increases down the group.The stability of + 6 oxidation state decreases down the group and stability of + 4 oxidation state increases (inert pair effect). Bonding in +4 and +6 oxidation states is primarily covalent.
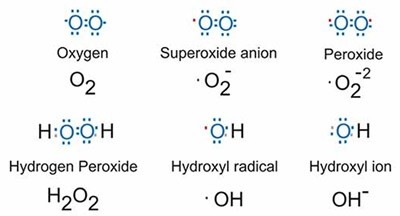
Since oxygen has very high electronegativity, it mainly shows only negative oxidation state as –2. However, in the case of OF2,it has an oxidation state of + 2. Likewise, apart from – 2 oxidation states, oxygen illustrates – 1 oxidation state in peroxides and – 1/2 oxidation state in superoxides. Oxygen also shows a positive oxidation state with compounds of fluorine. This happens because fluorine is more electronegative than oxygen. It shows +2 oxidation state in OF2 and +1 in O2F2.
Rest of the elements of the group, besides showing +2 oxidation states, also show +4 and +6 oxidation states owing to the availability of d-orbitals in their electronic structure.The reason why oxygen differs widely from the remaining elements of its group is because of its compact structure. It is small in size with high electronegativity and the nonattendance of d-orbitals in the valence shell. This means that it cannot exhibit more than 3 oxidation state due to its compatibility.
Another important point is that oxygen and sulphur have only s and p electrons, while the Se, Te, and Po have d and f electrons as well. The filling of d shell makes the atom smaller and hence the electrons are tightly packed. Other elements of the oxygen family exhibit + 2, + 4, + 6 oxidation states, however + 4 and + 6 are more common. Likewise, Se cannot acquire the highest oxidation state of (+VI). Also, when S is oxidized by HNO3 it produces H2SO4 (S +VI), but when Se is oxidized by HNO3 it forms H2SeO3 (Se +IV).
Sulphur, selenium and tellurium predominantly show + 4 oxidation state in their compounds with oxygen and oxidation state of + 6 with fluorine. Polonium barely shows –2 oxidation state due to increasing shielding effect.
In general terms, the chalcogen group can also share their two electrons with another element to form two covalent bonds. For example, H2O,F2O,Cl2O,H2S and SCl2. In these given examples, the chalcogen elements have a lower electronegativity than the corresponding element. For instance, in the case of SCl2where the electronegativity of chlorine is 3.5 and sulphuris2.5), then the oxidation state of sulphur is (+II).
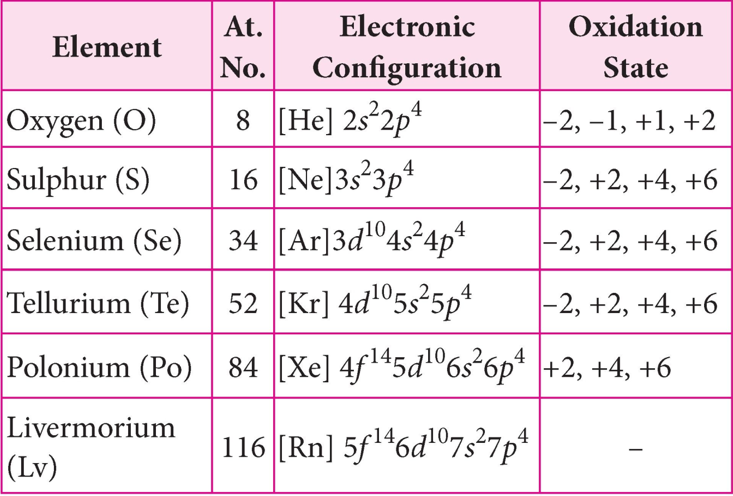
Fig : Oxidation states of group 16 elements.