Equilibrium: Strong and weak electrolytes
In a solution, a solute is a substance that dissolves in a solvent. Generally, the component present in the greatest quantity is the solvent. Water is the most common solvent because of its ability to dissolve almost any substance over time. When a solid dissolves into a liquid solvent such as water, competition occurs between the forces of attraction within the particles of the solute (solute-solute interactions) and the forces of attraction between the solvent molecules and the particles in the solute (solvent-solute interactions). The strength of interactions determines whether the solute dissolves.A concentrated solution contains a relatively large amount of solute than the solvent. A dilute solution contains a relatively small concentration of solute than the solvent. “Concentrated” and “dilute” aren’t much in quantity. Aqueous solutions have water as its solvent.
Arrhenius’s Theory of Electrolytic Dissociation put some light that certain substances dissociate into cations and anions when dissolved in water.These ions enables electricity to flow. The extent to which a solution can conduct an electric current directly depends on the number of ions present in the solution.An electrolyte is a substance that dissolved in water produces a solution that conducts electricity. These ions dissociate to form mobile, solvated cations and anions.
Figure 1: Electrical conductivity of aqueous solution can be shown with this electric circuit. The circuit will be only be completedand to allow electron flow when there are charge carriers (ions)in the solution.
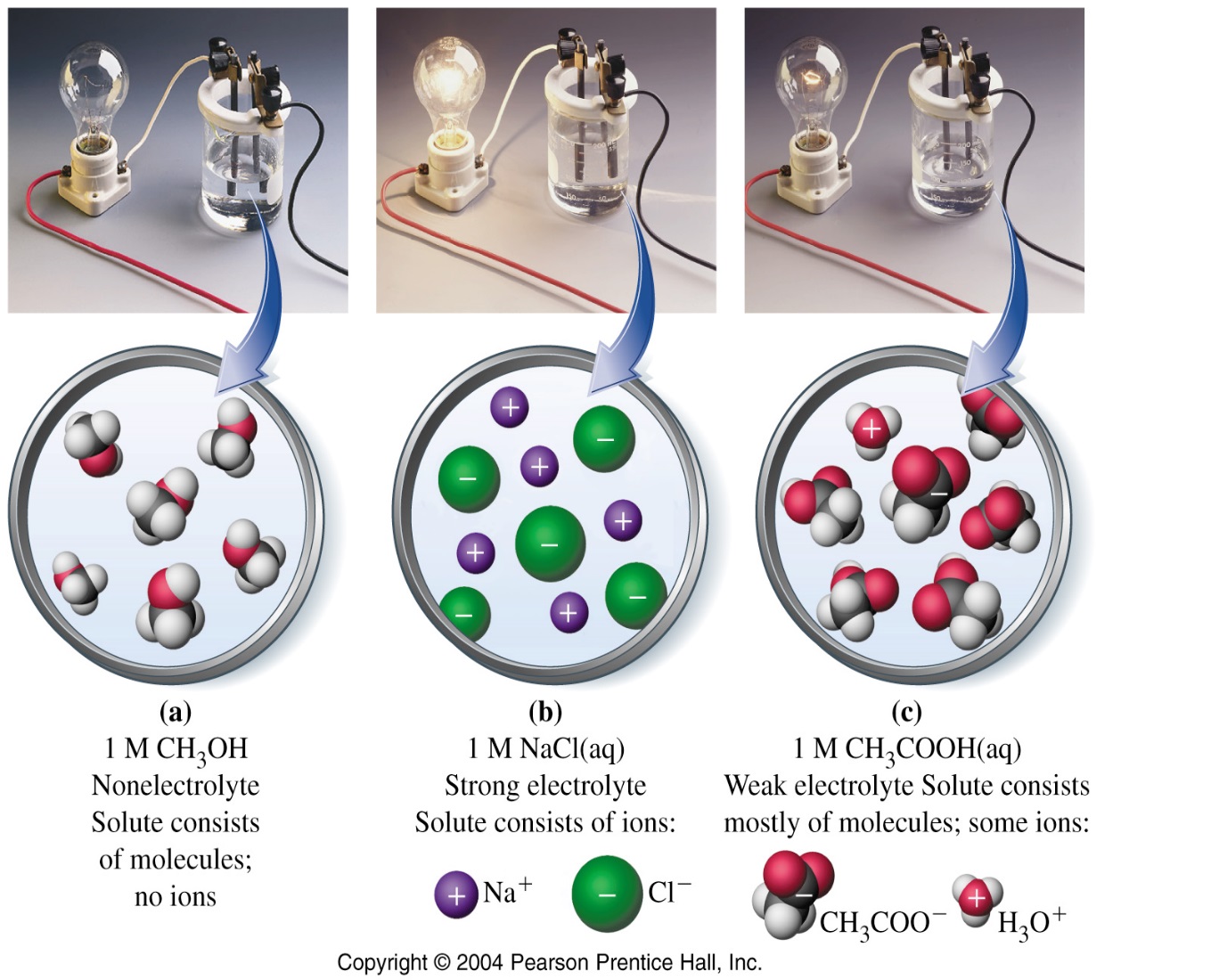
Ionic compounds dissociate into constituent ions when they are dissolved in solution. This is called dissociation. For example, When NaCl dissolves in water, it produces hydrated Na+ and Cl– ions in the solution. These ions present in the aqueous salt solution can conduct electricity strongly.
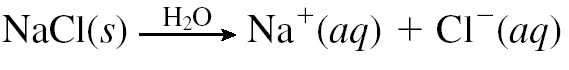
Formation of ions by molecular compounds when they are dissolved in water is called ionization. Substances acquire +ve/-ve charge b losing/gaining electrons. A strong electrolyte undergoes 100% dissociate in water. A strong electrolyte in a solution is available almost exclusively as ions.Virtually all the units of original solute are separated into ions. Strong electrolyte solutions are good conductors.Examples include all water-soluble ionic compounds, strong acids and strong bases.
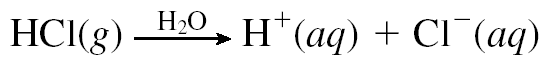
Weak electrolytes partially ionize in water. The mostly exist in molecular form. Examples include all weak acids and bases and a few ionic compounds.Weak electrolyte solutions are poor conductors.Different weak electrolytes dissociate to different extents.An ionic equilibrium is established in these electrolytes. Dissociation of weak electrolytes is often written using back-and-forth double arrows, indicating that adynamic equilibrium is taking place, in which there is a forward and a backward direction to the process.
Weak Base:
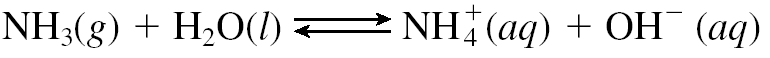
Weak acid:
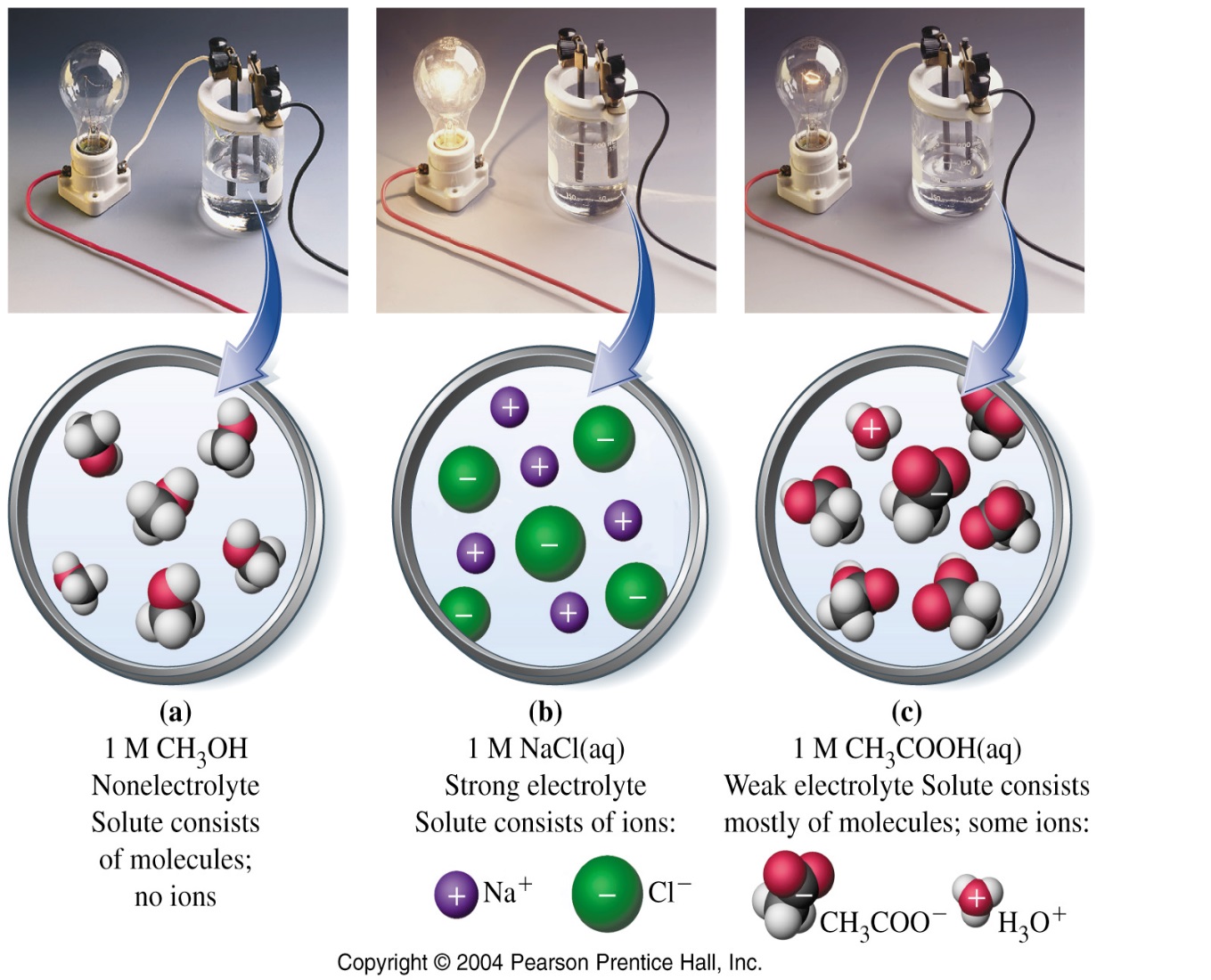
Alternatively, a non-electrolyte is a substance that certainly dissolved in water but produces a solution without ions. A nonelectrolyte is present in solution almost exclusively as molecules which are not capable of conducting electricity. Most molecular compounds and organic compounds are non-electrolytes. For example, the sugar solution cannot conduct electricity.
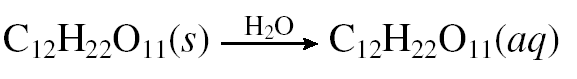
 |
 |
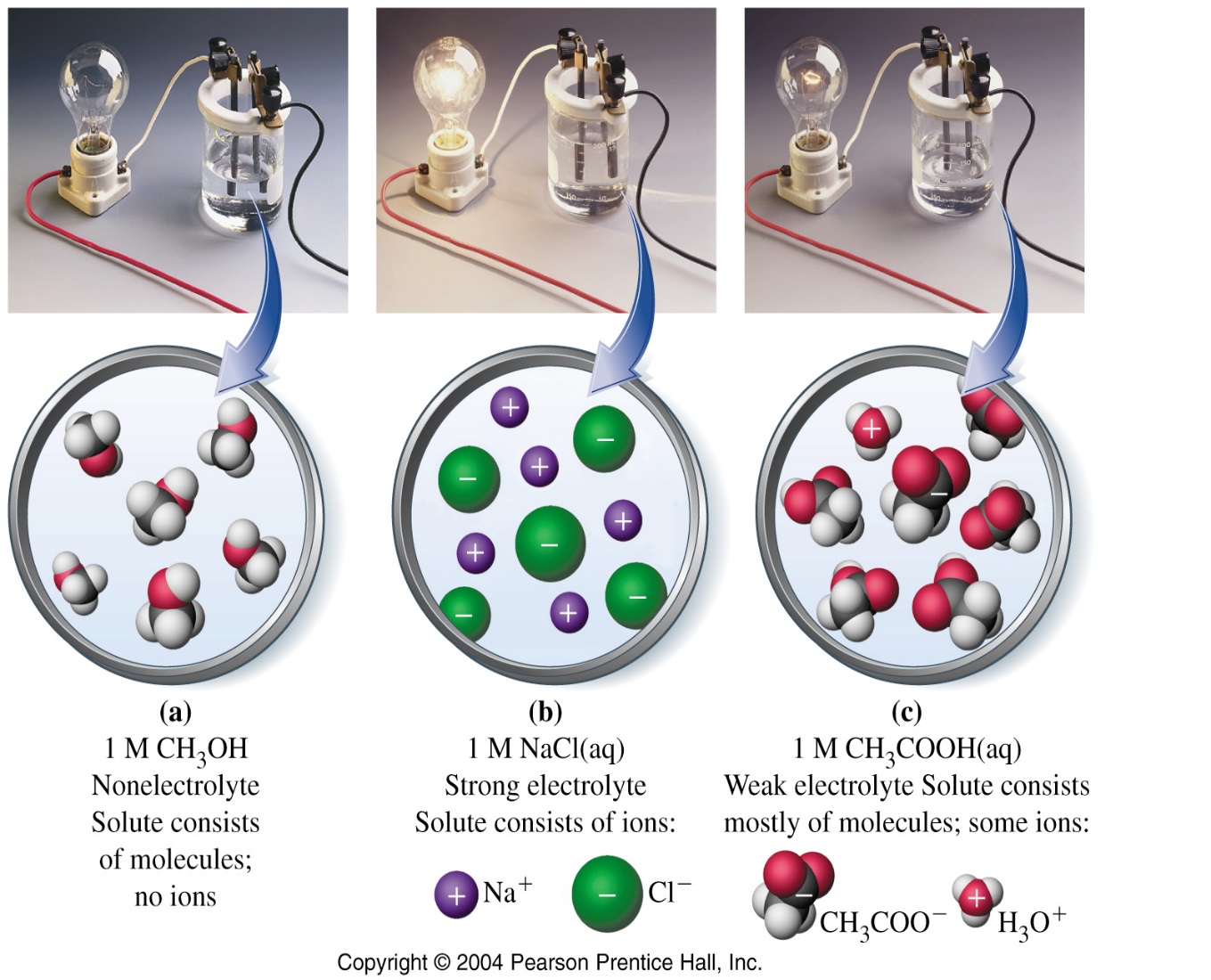 |
| Figure 2: Nonelectrolyte | Figure 3: Weak electrolyte | Figure 4: Strong electrolyte |