Surface tension refers to a physical property which is equal to the amount of force per unit area required to expand liquid’s surface. Surface tension is the tendency of the liquid surface which occupies the least possible surface area.
Surface tension is defined as the major factor in capillary action. Surfactants are defined as the extension of substances which can decrease liquid’s surface tension. For instance, mixing detergent in water reduces its surface tension. While black pepper sprayed on water floats, and the pepper along with detergent sprinkled on the water will be sunk.
Because of the intermolecular forces, forces of surface tension act between the molecules of the liquid at outer boundaries of liquid.
Force per unit length or energy per unit area are the units of surface tension.
Surface tension is defined as the work or energy which is needed to enhance liquid’s surface area because of intra-molecular forces. Since the inter-molecular forces acting on surface tension differs, which depends on liquid’s nature (such as water versus gasoline) or solutes present in the liquid (let’s say surfactants such as detergents), each solution display contradicts properties of surface tension.
It’s in your knowledge or not, but surface tension is a concept, you already seen at your work. At whatever time, you’ll fill a glass of water from very far; you might notice later that the water level in the glass is indeed greater than glass’s height.
Molecular Perspective
Two types of molecules act are there in the water sample. The molecules present on the inner surface are called the interior and the molecules present on the outer surface are called the exterior.
The interior molecules get attracted to the whole of molecules throughout them, and the exterior molecules get attracted only to the surface of other molecules and also attracted to those which are below the surface. This results in the difference in energy state/energy levels as the the energy level of the molecules present in the interior is very much lower than the molecules acting in the exterior part.
Due to this, molecules attempt to balance out a minimum surface area and hence allow greater molecules to consist of lower energy state. This is what defined at the surface tension. An illustration of surface tension has been given in the figure below:
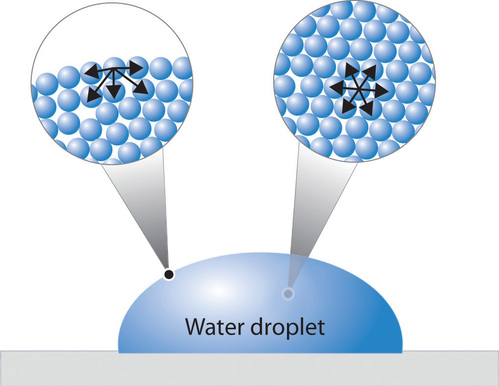
At the surface of the water, the molecules present, experience a pure attraction to other molecules found in liquid which grip the bulk sample surface together. On contrary, interior surface experience unchanging attractive forces.
Due to the polar properties of water, water molecules attract each other. Hydrogen ends, which are positive compared to the negative ends of O atom, and cause water for “sticking” together. This is the reason that surface tension occurs and it takes a specific amount of energy to break or separate these inter-molecular bonds.
The same phenomenon is applied to other liquids, also to hydrophobic liquids like oil. These forces act between the liquid like Van der Waals forces which are responsible for inter-molecular forces formed in the liquid.
After this, it will take a particular amount of energy to break the surface tension and these intermolecular forces. Water is that liquid which is having a pretty high value of surface tension and plus it’s a tough task to overcome.
Things can float because of the surface tension of the water, which is denser than the water and thus, allows organisms or creatures to literally walk on the water surface. (Fig. 2).
Surface tension even allows the water droplet formation that we get to see in nature.
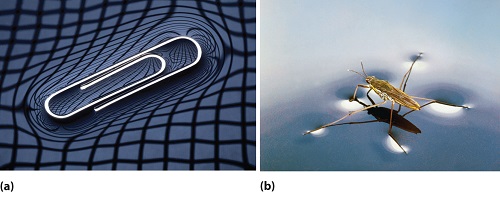
Fig. 2